Hepatocellular carcinoma (HCC) is the most widespread form of liver cancer, and it is believed to be one of the leading causes of cancer-related deaths on earth. The sixth most common malignancy and the third most common cause of death due to cancer in the world is liver cancer. The most common of these is hepatocellular carcinoma, with approximately 75 percent of liver cancer cases [1]. The epidemiology of liver cancer has changed considerably over the past few decades. Between 1978 and 2012, the incidence of primary liver cancer declined in some Asian countries and in Italy, but increased in India, the Americas, Oceania, and many parts of Europe [2]. Patients with advanced liver cancer have a low number of choices in treatment and a poor survival rate in general [3, 4]. These disparities are mainly due to the variation in the hepatitis prevalence, the level of alcohol intake, obesity, and other metabolic disorders, which have led to a continuous burden of liver cancer in the world.
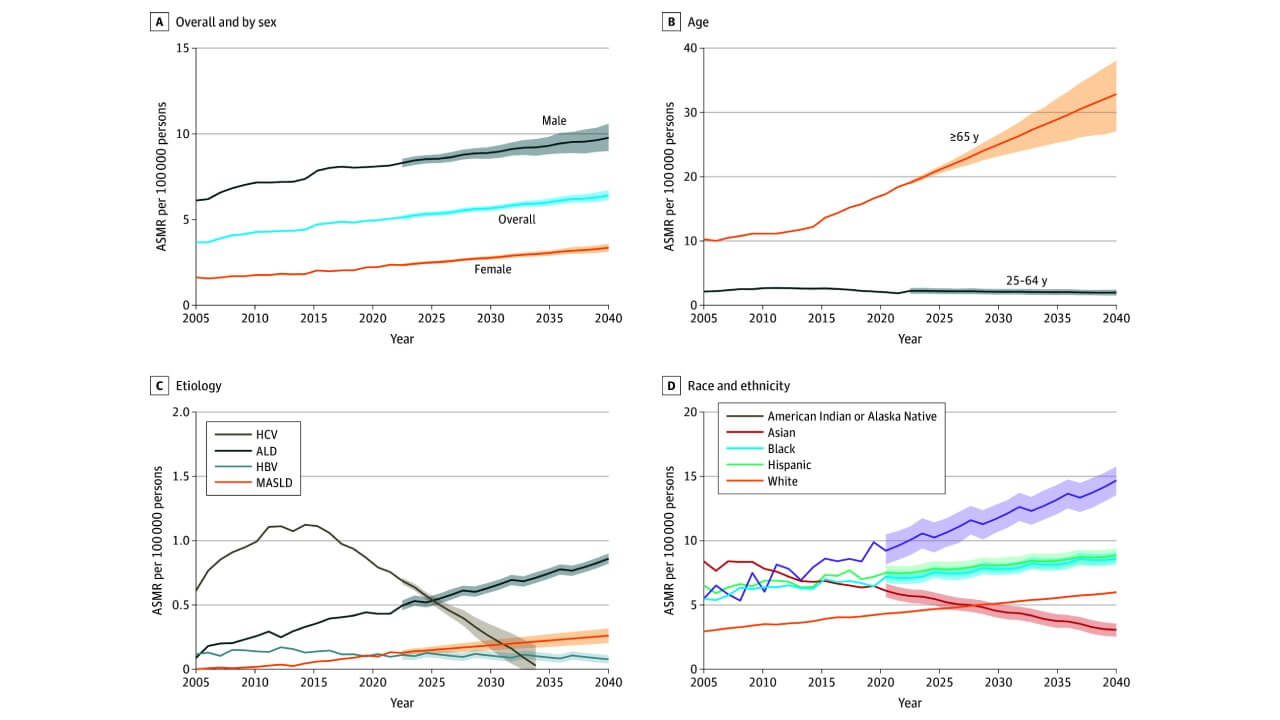
In the previous years, Germany has come to the fore in the development of new approaches for treating liver cancer patients, especially those with advanced hepatocellular carcinoma (HCC). Conventional therapies, such as surgery, targeted therapy, chemotherapy, and local ablation, are still used. But we can see, the systemic therapy environment is changing fast with the emergence of immunotherapy. The use of immunotherapy in treating HCC is a new hope for patients who haven't had it before. Combined with cancer vaccines and targeted therapies, in addition to the stimulation of immune cells and the improvement of the immune response, immune checkpoint inhibitors have revolutionized medical oncology, allowing new treatment options. To reach the most recent standards of individualized cancer therapies, professionals in Germany are more and more adopting immunotherapy combination regimes in order to enhance the results in early-stage as well as advanced HCC.
Causes, Risk Factors, and Disease Progression
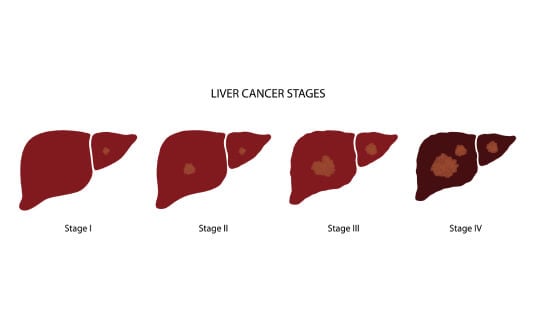
Liver cancer is a very complex and aggressive form of cancer that occurs when abnormal cells grow uncontrollably and develop into malignant tumors. This disease can begin in the liver tissue, and then it is called primary liver cancer, or it can occur in the form of secondary spread as metastatic cancer. Hepatocellular carcinoma (HCC) is the most common type of liver cancer, accounting for nearly 75% of all cases worldwide. Despite the breakthroughs in the diagnosis and prevention of liver cancer, patients diagnosed with advanced liver cancer face severe challenges due to the imperceptible nature of the disease and its resistance to conventional therapy. To prevent the development of liver cancer and diagnose it at the initial stages, one must be aware of the mechanisms of its formation and the most severe risk factors that lead to it.
How Liver Cancer Develops
The regenerative activity of liver cells is compromised by the chronic destruction of this essential organ caused by viral diseases, alcoholism, or fatty liver disease. In the long run, the constant inflammation and regeneration may result in a genetic alteration of cells that may become cancer cells.
The primary liver cancer originates from hepatocytes or the bile duct, but the advanced hepatocellular carcinoma (HCC) can spread to blood vessels and other organs. Metastatic liver cancer originates from tumor cells in other organs, such as the colon, lungs, or breast, which spread to the liver through the bloodstream. Once in the liver, cancer cells can bypass the immune system, making them difficult to treat. The current therapies, such as immunotherapy, are designed to increase the capability of the immune system to detect and destroy such cancer cells.
Main Risk Factors for Liver Cancer
Various factors known to increase the risk of liver cancer are primarily those associated with long-term liver damage. Chronic hepatitis B/C infections, chronic alcoholism, and obesity-related non-alcoholic fatty liver disease are the most prevalent ones [6]. Patients who have cirrhosis, whose normal liver tissue has been substituted with scar tissue, are especially prone to the development of primary liver cancer or advanced liver cancer.
Other causes are exposure to aflatoxins (poisonous substances produced by some fungi in food), smoking, and some hereditary metabolic disorders. There is also a genetic and gender factor, as men are more prone to liver cancer than women, partly due to hormonal differences and lifestyle. The disease progression can be prevented by early intervention and screening of high-risk groups, which allows more efficient treatment approaches used in German cancer centers.
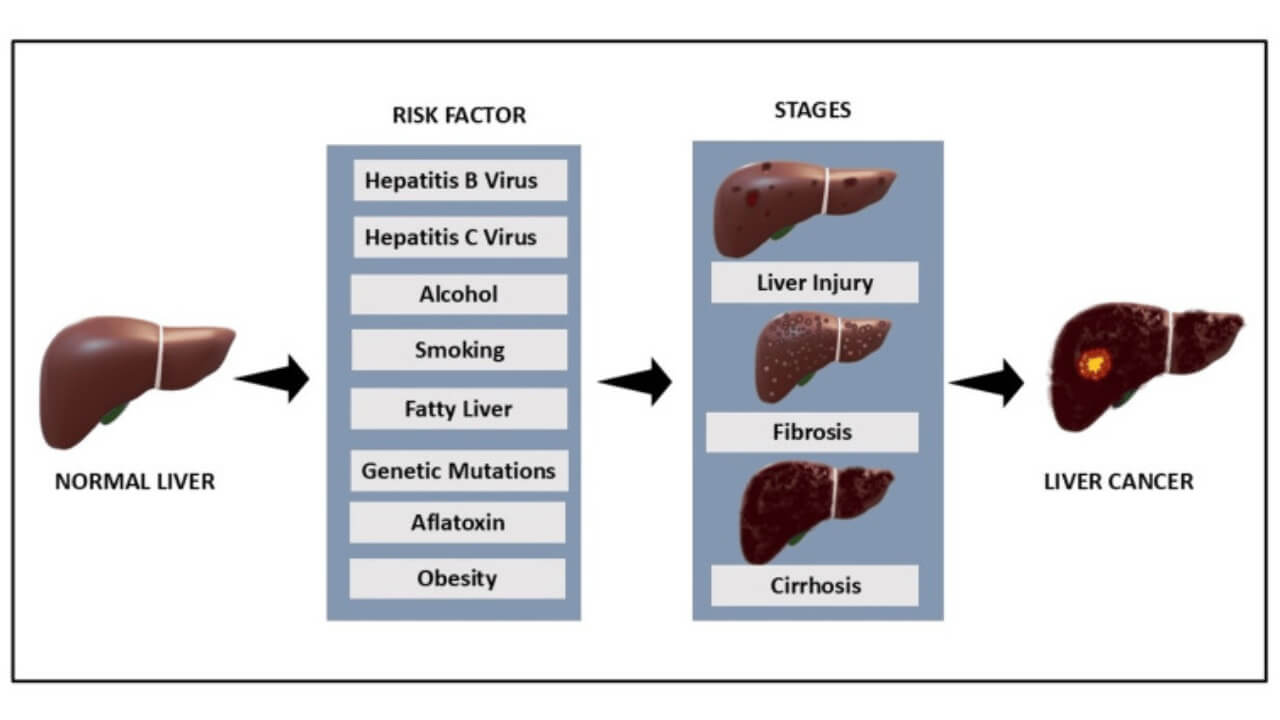
How Liver Cancer Progresses
The stages of liver cancer progression follow specific phases. At first, the small tumors can be localized and asymptomatic, which is commonly known as early-stage HCC. Later in the progression of the disease, the cancer cells spread to the surrounding blood vessels and tissues, resulting in progressive liver cancer or progressive hepatocellular carcinoma. This cancer can suppress the immune cells and weaken the normal defenses of the body at this stage, and in most cases, the immune system is not able to control the tumor growth.
This step brings out the reason why immunotherapy for liver cancer has become vital in the field of modern medical oncology. The proposed new treatments are expected to restore the immune system, trigger the tumor-infiltrating lymphocytes and natural killer cells, and enhance the immune system in such a manner that it is able to attack and destroy malignant cells. Immunotherapy combinations are becoming increasingly popular among clinicians in Germany for HCC treatment, which can now offer better long-term control in even advanced liver cancer.
Benefits of Immunotherapy for Liver Cancer Patients
Over the past decade, immunotherapy has transformed the approach to treating liver cancer, particularly for patients with advanced liver cancer or those who are not candidates for surgery. The authoritative approach to this novel type of systemic treatment is that it utilizes the body's immune system to identify and destroy malignant cells to provide a remedial approach that goes beyond the destruction of tumors. Unlike chemotherapy, which destroys a specific target—fast-growing cancer cells—immunotherapy for liver cancer trains and directs the body's defense mechanisms to mount a more targeted and prolonged immune attack.
German medical oncology centers have been at the forefront in using the immunotherapy combination regimens, which incorporate immune checkpoint inhibitors, cancer vaccines, and adoptive transfer of tumor-infiltrating lymphocytes. These methods are meant to prompt immune cells like T-lymphocytes and natural killer cells to infiltrate cancer cells and inhibit their development [7]. Immunotherapy is used to stimulate the recognition of cancer cells as foreign to allow the immune system to overcome the immunosuppressive microenvironment that characterizes progressing hepatocellular carcinoma. Consequently, the patients have better long-term disease control in addition to tumor regression, which explains why immunotherapy forms a cornerstone of the management of liver cancer.
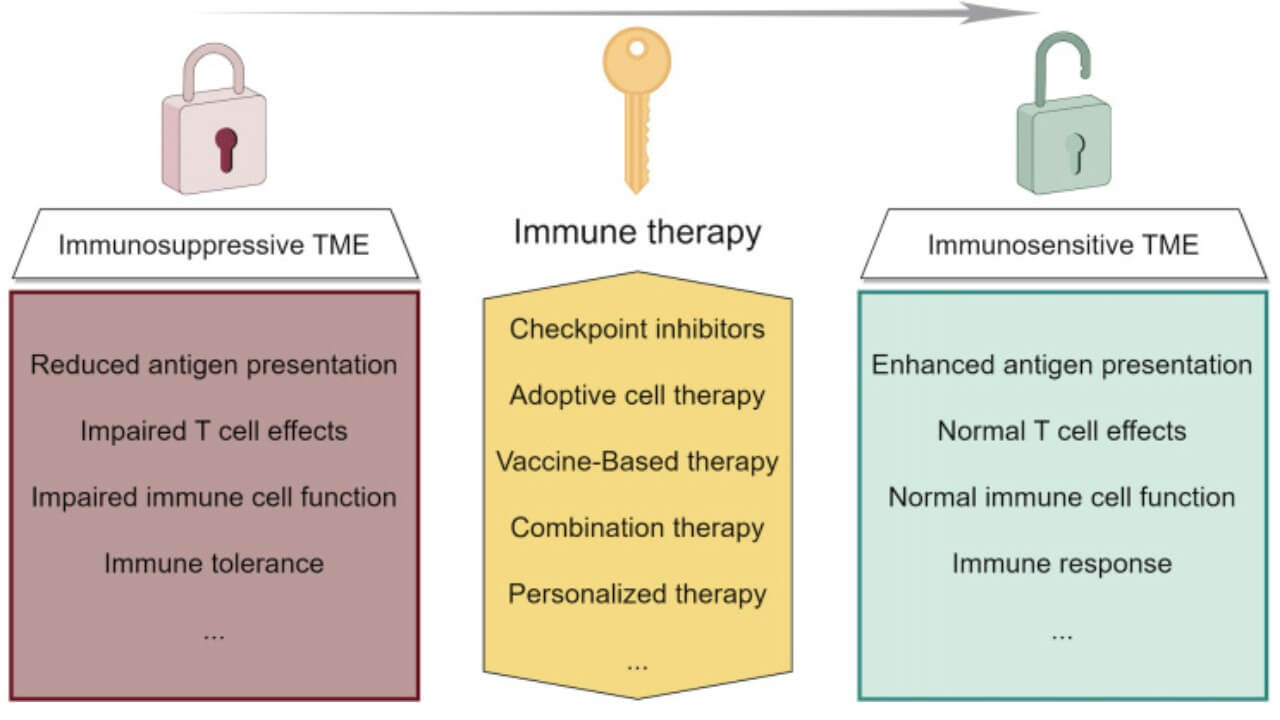
How Immunotherapy Works in Liver Cancer
When the cancer cells evade the immune system, the immune system is unable to provide a proper defense. Immunotherapy helps in this by increasing the sensitivity of the immune cells, boosting the immune response, and allowing them to better eliminate cancerous cells. New therapies, including cancer vaccines and immune checkpoint inhibitors, prime the immune system to identify tumor-specific antigens in order to sustain long-term immunity against relapse.
This can be used in primary liver cancer, whereby the tumor is formed out of the hepatocytes and can be used to avoid the spreading of cancer cells to other parts of the liver. Combination treatment that includes immunotherapy is progressively utilized as first-line treatment for advanced HCC in the form of monotherapy or in combination with targeted therapies to attain prolonged clinical response. Such immunotherapy combination regimens have demonstrated encouraging results, as they prolong patient survival and enhance the quality of life, even in some previously incurable cases.
Why Immunotherapy Is Especially Effective for Liver Cancer
The gastrointestinal tract constantly exposes the liver to antigens, which makes the liver unique and complex in its immune environment. This constant exposure may sometimes weaken the immune system, which enables the tumor cells to proliferate without detection. The disease is treated with immunotherapy, which activates immune cells and induces a strong, long-term immune response in the liver. German experts can use immunotherapy in combination with other treatments to make cancer cells more sensitive to therapy, shrink tumors, and prevent the development of advanced hepatocellular carcinoma.
The other benefit of immunotherapy is that it gives long-term results. Once the immune system is trained to recognize and target specific cancer cells, it provides ongoing surveillance to prevent recurrence and metastasis. This long-lasting protective mechanism renders immunotherapy a superior treatment compared to conventional therapies, which tend to lose their effectiveness after the treatment duration is over.
Key benefits of immunotherapy for liver cancer patients include:
- Enhanced treatment options. Immunotherapy is a new hope for patients who are unable to undergo surgery or fail to respond to traditional treatment, as it offers an alternative method of treatment involving the use of the natural body defense and the immune system.
- Reduced side effects. Immunotherapy has fewer and milder side effects compared to traditional chemotherapy, and patients can enjoy a better quality of life throughout the treatment process.
- Long-term protection. Immunotherapy can promote cancer prevention by training the immune system to attack cancerous cells and therefore offer long-term protection against cancer recurrence, which may prolong life expectancy compared to conventional therapies.
- Combination potential. Immunotherapy can always be used effectively alongside other modes of treatment, like surgery or targeted therapy, to optimize the curative effects and enhance the overall success of a treatment.
Types of Immunotherapy Used for Liver Cancer in Germany
The leading country in the high-level immunotherapy of liver cancer is Germany, which uses certain therapies that specifically attack the tumor microenvironment (TME). TME is the intricate network of cells, signaling molecules, and blood vessels that envelop and feed malignant tumors [8]. This microenvironment tends to inhibit the immune response of the body, so cancer cells can escape the immune surveillance. Immunotherapy of liver cancer is aimed at overcoming this immunosuppression, stimulating the immune cells, and restoring the natural antitumor immune response.
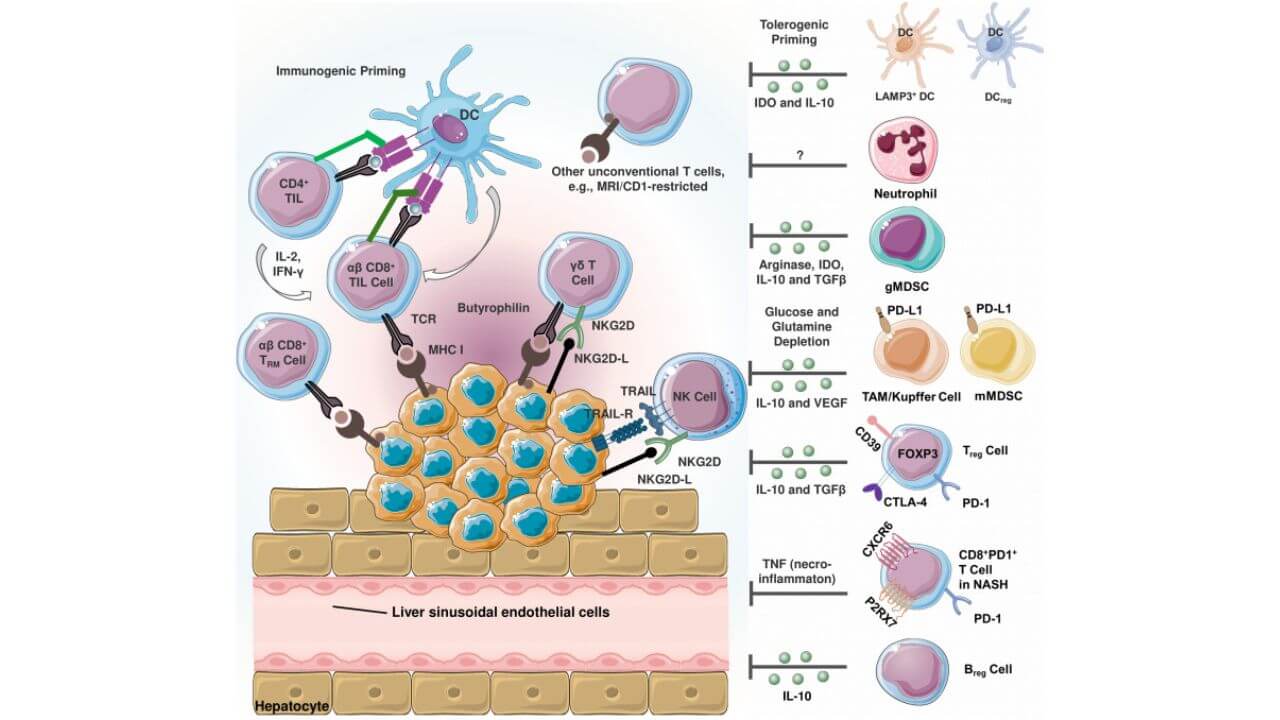
In case of progressive hepatocellular carcinoma, German researchers use immune checkpoint blockers, dendritic cell vaccines, and adoptive cell therapeutic methods together with targeted therapy or chemotherapy to construct comprehensive immunotherapy combination regimens. These advances have established a new paradigm in medical oncology, as modern protocols have significantly improved both survival and quality of life in patients with previously untreatable forms of primary liver cancer.
Immune Checkpoint Inhibitors
Immune checkpoint inhibitors are one of the most beneficial systemic treatment agents for primary liver cancer and advanced HCC compared to other modern treatment methods. The drugs focus on immune checkpoints, internal controls that occasionally stop the immune cells from attacking the tumor cells. By blocking these checkpoints, the drugs also restore a strong immune response, allowing the body's immune system to effectively destroy cancer cells.
It has been proven that immunotherapy together with targeted therapy can induce a greater response rate than traditional chemotherapy or surgery. This combination is now used as a first-line treatment for advanced hepatocellular carcinoma, providing better tumor control in patients with aggressive disease and thereby increasing survival.
Cancer Vaccines
A more advanced form of personalized medicine is the use of dendritic cell vaccines as one of the recent advancements in immunotherapy for the treatment of liver cancer. Dendritic cells are immune cells that are specialized to mediate between the adaptive and innate immune systems. They develop antigens that are similar to cancer cells and attach them to T-lymphocytes, thus causing a tumor-specific immune response. The fundamental role of dendritic cells in initiating immune responses was recognized with the Nobel Prize in Physiology or Medicine in 2011.
Dendritic cell vaccines are made patient-specific in German clinics. Blood samples are taken, and immature dendritic cells are isolated and trained in the laboratory with tumor-associated antigens of the cancer cells. These dendritic cells are reinfused once they are mature, so the patient's immune system can attack malignant cells with high precision.
This has demonstrated the good results in both metastatic and primary liver cancer, especially among patients who are not responsive to chemotherapy or targeted therapies. Also, it can be used to prevent recurrence as it stimulates tumor-infiltrating lymphocytes and natural killer cells. As a result, the dendritic cell vaccines are now considered one of the most promising long-term cancer control modalities.
Adoptive Cell Therapy
One of the most personalized immunotherapies for liver cancer patients is adoptive cell therapy (ACT). The procedure involves removing the immune cells of the patient, growing them in the laboratory, and reintroducing them into the body to help boost the immune system. The two commonest forms of ACT are the tumor-infiltrating lymphocytes and natural killer cells, which play a significant role in locating and eliminating cancerous cells.
Adoptive cell therapy (ACT) is a common treatment for high-grade liver cancer or advanced HCC in Germany and is usually combined with targeted therapies or immune checkpoint inhibitors. Such a combination improves the work of the immune system and makes it possible to provide long-term immune control after the treatment. Patients undergoing these therapies have shown significant tumor regression and extended remission, especially following failure of conventional therapies.
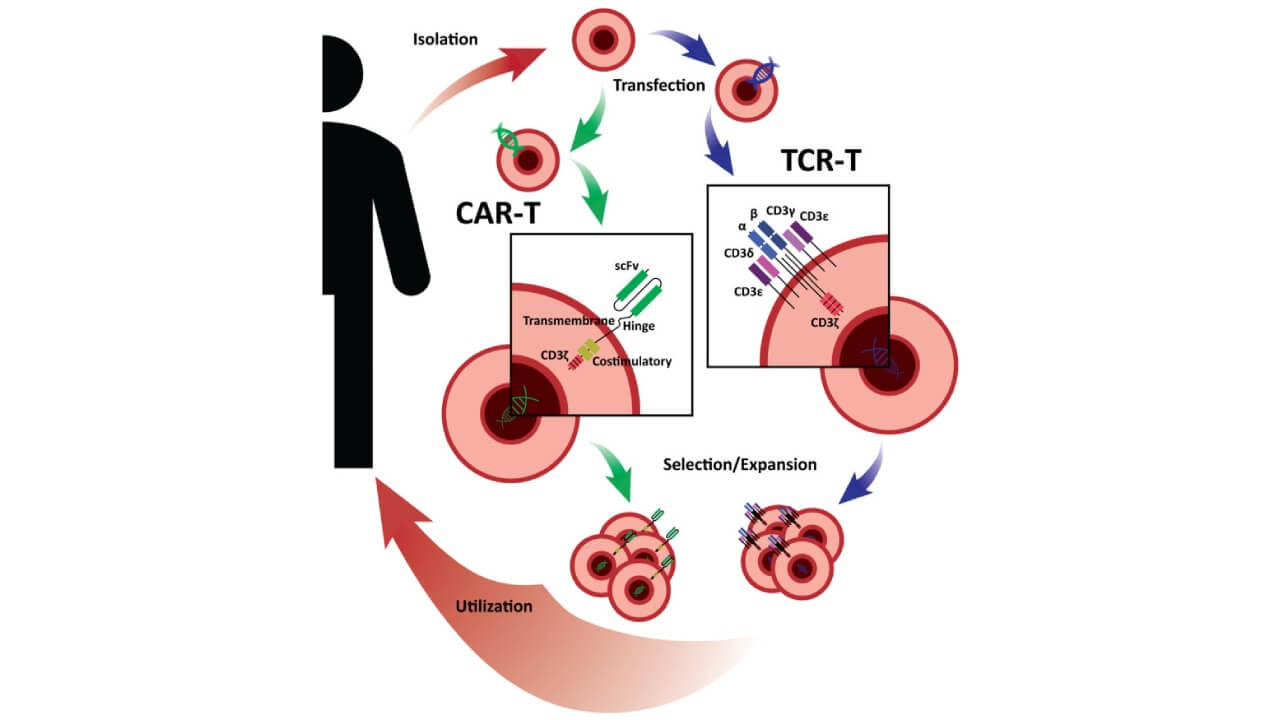
Cytokine and Immune-Modulating Therapy
In certain regimens of immunotherapy for liver cancer, natural signaling proteins are also used. Interleukin-2 (IL-2), interferons, and other immune modulators influence and boost the immune response against cancer. These treatments may be used as complements to targeted therapies or chemotherapy to increase antitumor efficacy without affecting the safety profile.
Cytokine therapy is especially useful in treating patients who cannot receive chemotherapy or surgery, which is an alternative treatment where the body can easily find an immunological solution. New research is underway to refine cytokine-based immunotherapy to maximize the activation of immune cells at minimal levels of toxicity.
Combination Approaches
The combination immunotherapy regimens have the greatest effects on the treatment of liver cancer. The strategies involve the application of immunotherapy with targeted therapies, chemotherapy, and local ablation. This type of combination has become the new standard of care in the treatment of advanced hepatocellular carcinoma, as it addresses the various tumor development pathways.
The synergistic interaction of immunotherapy and targeted therapy allows German oncologists to attack tumor cells in two different ways: directly destroying malignant cells and activating the immune system to support an anti-tumor response. In this way, it is possible to treat cancer more accurately and effectively, extending the lifespan and increasing the quality of patients' lives at various stages of the disease, starting with early-stage liver cancer and ending with advanced liver cancer.
| Treatment | 2-Year Survival Rate* | Response Rate** | Cost of treatment,€*** |
|---|---|---|---|
| Dendritic cell therapy | up to 85% | up to 90% | 20,000 - 38,000 |
| Checkpoint inhibitors | up to 45% | up to 45% | 375,000 - 420,000 for the full course |
| CAR T-cell therapy | up to 50% | up to 40% | 450,000 - 550,000 |
*Booking Health data. Survival rates vary widely depending on the type of cancer, the patient's condition, and specific circumstances.
**Booking Health data. Response rate is the number of patients who show improvement after treatment.
***The cost of treatment depends on the type of tumor, stage of cancer, and other factors.
Leading Clinics and Experts for Liver Cancer Immunotherapy in Germany
In Germany, immunotherapy for liver cancer of all stages is provided, and the treatment depends on the individual situation. The size of the tumor, the stage of the disease, and the condition of the immune system are some of the factors considered by the oncologists of the specialized medical centers to identify the best immunotherapy process.
Several leading German centers have pioneered innovative immunotherapy approaches:
LDG Laboratories Dr. Gansauge Berg
This center was one of the first to use LANEX-DC, a dendritic cell vaccine, and has already done more than 2,500 procedures. The clinic deals with advanced liver cancer and other primary liver cancers and provides individualized dendritic cell-based therapies to stimulate the immune response with minimal side effects. Therapies are done on an outpatient basis, in combination with other treatments.
Asklepios Hospital Barmbek Hamburg
One of the leading medical tourism centers in the world, according to the German Cancer Society. Reputed academic hospital that treats more than 110,000 patients a year. The center uses several combination approaches, where immunotherapy, targeted therapy, and conventional therapies are combined to enhance the ability of immune cells to fight cancer and maintain the disease at long-term control.
Clinic of Advanced Biological Medicine Frankfurt
This clinic specializes in integrative cancer care involving the use of conventional oncology and advanced biological therapies. The method has more than 40 years of experience and is aimed at strengthening the immune system and reducing the side effects of first-line treatment. The protocols were developed to provide effective immunity in patients with primary liver cancer or advanced HCC.
Hyperthermia Center Munster
This center employs innovative strategies combining immunotherapy with six hyperthermia techniques in an outpatient setting. The approach aims to enhance both immune cell activity and tumor targeting, supplementing targeted therapy for advanced HCC to improve both survival and quality of life.
A Medical Journey: Every Step of the Way With Booking Health
Finding the best treatment strategy for your clinical situation is a challenging task. Being already exhausted from multiple treatment sessions, having consulted numerous specialists, and having tried various therapeutic interventions, you may be lost in all the information given by the doctors. In such a situation, it is easy to choose a first-hand option or to follow standardized therapeutic protocols with a long list of adverse effects instead of selecting highly specialized innovative treatment options.
To make an informed choice and get a personalized cancer management plan, which will be tailored to your specific clinical situation, consult medical experts at Booking Health. Being at the forefront of offering the latest medical innovations for already 12 years, Booking Health possesses solid expertise in creating complex management programs in each individual case. As a reputable company, Booking Health offers personalized treatment plans with direct clinic booking and full support at every stage, from organizational processes to assistance during treatment. We provide:
- Assessment and analysis of medical reports
- Development of the medical care program
- Selection of a suitable treatment location
- Preparation of medical documents and forwarding to a suitable clinic
- Preparatory consultations with clinicians for the development of medical care programs
- Expert advice during the hospital stay
- Follow-up care after the patient returns to their native country after completing the medical care program
- Taking care of formalities as part of the preparation for the medical care program
- Coordination and organization of the patient's stay in a foreign country
- Assistance with visas and tickets
- A personal coordinator and interpreter with 24/7 support
- Transparent budgeting with no hidden costs
Health is an invaluable aspect of our lives. Delegating management of something so fragile yet precious should be done only to experts with proven experience and a reputation. Booking Health is a trustworthy partner who assists you in pursuing stronger health and a better quality of life. Contact our medical consultant to learn more about the possibilities of personalized treatment with innovative methods for different types of cancer with leading specialists in this field.
International Cancer Care: Patient Stories with Booking Health
FAQ: Immunotherapy treatment for liver cancer
Send request for treatmentLiver cancer patients have a variable life expectancy based on the stage of the disease, general health, and the chosen treatment. Early diagnosis and the availability of treatment measures such as immunotherapy or targeted therapy can increase survival.
The appropriate treatment would be based on the stage and type of liver cancer. The possible treatments are surgery, chemotherapy, targeted therapy, and immunotherapy, which in most cases are combined to ensure the tumors are well controlled and the disease is managed in the long term.
It depends on the response and the protocol of the treatment. Patients with advanced liver cancer can often have a limited survival rate.
In Germany, several types of immunotherapy are available to treat liver cancer in cancer facilities, and they are checkpoint inhibitors, dendritic cell vaccines, and adoptive cell therapy.
Checkpoint inhibitors for liver cancer are promising in their results, particularly in advanced HCC. These medications replenish the immune cells against cancerous cells, enhancing survival and providing an option in instances where the conventional therapies are not effective.
Dendritic cell vaccines in liver cancer therapies are designed to educate the immune system. These vaccines help destroy cancer cells and promote long-term protection against recurrence by enabling the activation of T-cells and natural killer cells.
Yes, adoptive cell therapy for liver cancer isolates and clones immune cells of a patient and then replenishes them. It is effective against tumor cells, boosts the immune system, and is particularly applicable for advanced hepatocellular carcinoma or cases that are not responsive to other therapies.
PD-L1 is a biomarker that can be used to predict response to immunotherapy. This indicator directs individual treatment planning and enhances liver cancer outcomes.
Patients with any stage of liver cancer may be candidates for dendritic cell treatment. German centers evaluate the previous treatment, health status, and biomarkers, and provide a choice of immunotherapy for liver cancer.
The Booking Health support for immunotherapy in Germany covers consultations, hospital choice, travel arrangements, and documentation. They facilitate access to checkpoint inhibitors, dendritic cell vaccines, and adoptive cell therapy for liver cancer in the leading German clinics.
Germany offers surgery, targeted therapy, chemotherapy, local ablation and immunotherapy. Immunotherapy and combination regimens provide additional options for patients who cannot undergo surgery often improving survival and quality of life.
What types of immunotherapy are used for liver cancer treatment in Germany, and how do they differ in effectiveness?
Common types include immune checkpoint inhibitors, dendritic cell vaccines, adoptive cell therapy, and cytokine therapy. Effectiveness varies but combination approaches offer the best long-term disease control.
Costs depend on therapy and cancer stage. Dendritic cell therapy: €20,000–38,000; checkpoint inhibitors: €375,000–420,000; CAR T-cell therapy: €450,000–550,000 for the full course.
Dendritic cell therapy shows the highest response rates, up to 85% 2-year survival and 90% response, especially when combined with other treatments to boost immune attack on tumors.
In Germany liver cancer patients can access full spectrum of immunotherapy options. Treatments are individualized and administered in specialized centers. Also they are combined with standard oncology care to enhance immune response.
Choose treatment abroad and you will for sure get the best results!
Authors:
This article was edited by medical experts, board-certified doctors Dr. Nadezhda Ivanisova, and Dr. Bohdan Mykhalniuk. For the treatment of the conditions referred to in the article, you must consult a doctor; the information in the article is not intended for self-medication!
Our editorial policy, which details our commitment to accuracy and transparency, is available here. Click this link to review our policies.
Sources:
[1] Jessica L Petrick, Andrea A Florio, Ariana Znaor et al. International trends in hepatocellular carcinoma incidence, 1978-2012. Int J Cancer. Author manuscript; available in PMC: 2021 Jul 15. Published in final edited form as: Int J Cancer. 2019 Nov 5;147(2):317–330. doi: 10.1002/ijc.32723. Front Endocrinol (Lausanne). 2022 Jul 8;13:860671. doi: 10.3389/fendo.2022.860671. [DOI] [PMC free article]
[2] Patricia C Valery, Mathieu Laversanne, Paul J Clark et al. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology. 2018 Feb;67(2):600-611. doi: 10.1002/hep.29498. Epub 2017 Dec 23. [DOI] [PubMed]
[3] Jessica L Petrick, Andrea A Florio, Rohit Loomba, Katherine A McGlynn. Have incidence rates of liver cancer in the US peaked? Cancer. Author manuscript; available in PMC: 2021 Jul 1. Published in final edited form as: Cancer. 2020 Apr 15;126(13):3151–3155. doi: 10.1002/cncr.32794. [DOI] [PMC free article]
[4] Xiaotao Zhang, Hashem B El-Serag, Aaron P Thrift. Sex and Race Disparities in the Incidence of Hepatocellular Carcinoma in the United States Examined through Age-Period-Cohort Analysis. Cancer Epidemiol Biomarkers Prev. 2020 Jan;29(1):88-94. doi: 10.1158/1055-9965.EPI-19-1052. Epub 2019 Nov 11. [DOI] [PubMed]
[5] Sikai Qiu, Jiangying Cai, Zhanpeng Yang et al. Trends in Hepatocellular Carcinoma Mortality Rates in the US and Projections Through 2040. JAMA Netw Open. 2024 Nov 18;7(11):e2445525. doi: 10.1001/jamanetworkopen.2024.45525. [DOI] [PMC free article]
[6] Shivani R Kale, Geeta Karande, Anand Gudur et al. Recent Trends in Liver Cancer: Epidemiology, Risk Factors, and Diagnostic Techniques. Cureus. 2024 Oct 23;16(10):e72239. doi: 10.7759/cureus.72239. [DOI] [PMC free article]
[7] Yanju Liu, Hongyuan Yang, Tian Li, Na Zhang. Immunotherapy in liver cancer: overcoming the tolerogenic liver microenvironment. Front Immunol. 2024 Sep 3;15:1460282. doi: 10.3389/fimmu.2024.1460282. [DOI] [PMC free article]
[8] Zeynep Akbulut, Başak Aru, Furkan Aydın, Gülderen Yanıkkaya Demirel. Immune checkpoint inhibitors in the treatment of hepatocellular carcinoma. Front Immunol. 2024 Apr 4;15:1379622. doi: 10.3389/fimmu.2024.1379622. [DOI] [PMC free article]
[9] Muhammet Ozer, Suleyman Yasin Goksu, Baran Akagunduz, Andrew George, Ilyas Sahin. Adoptive Cell Therapy in Hepatocellular Carcinoma: A Review of Clinical Trials. Cancers (Basel). 2023 Mar 16;15(6):1808. doi: 10.3390/cancers15061808. [DOI] [PMC free article]
Read:
Cancer immunotherapy in Germany
Innovative solutions for the treatment of liver cancer and liver metastases
Dendritic cell therapy in cancer treatment in Germany - Vaccination against cancer
Article menu:
- Causes, Risk Factors, and Disease Progression
- Benefits of Immunotherapy for Liver Cancer Patients
- Types of Immunotherapy Used for Liver Cancer in Germany
- Leading Clinics and Experts for Liver Cancer Immunotherapy in Germany
- A Medical Journey: Every Step of the Way With Booking Health
- FAQ: Immunotherapy treatment for liver cancer
Don't know where to start?
Contact Booking Health