Cervical cancer affects approximately 660,000 women worldwide each year, making it the fourth most common cancer among women globally [1]. While early detection typically offers excellent cure rates, advanced cases require innovative treatment approaches. Dendritic cell therapy represents a promising personalized immunotherapy option, where specialized vaccines are created using a patient's own immune cells to strengthen the body's natural cancer-fighting response [2]. This treatment is showing encouraging results in clinical practice and is available at select medical centers. For patients seeking advanced treatment options, the Booking Health service provides access to experienced specialists at German hospitals specializing in treating cervical cancer with dendritic cells.
What are dendritic cells?
Immunotherapy for cancer is considered one of the most promising areas of medicine. It is with this that the upcoming breakthrough in cancer treatment is associated. Some immunotherapy methods have already proven themselves to be effective, allowing for the suppression of the development of cancer for a long time or even curing cancer in advanced stages.
Dendritic cells (DC cells) are used to stimulate an antitumor immune response. They are different immune cells that "learn" the antigen themselves and then "train" the T cells to fight the foreign agent in the body. American scientist Ralph Steinman's discovery of dendritic cells earned him the Nobel Prize in Medicine in 2011, highlighting the profound impact this research has had on cancer treatment development.
Dendritic cells are considered the most powerful specialized antigen-presenting immune cells. They have the unique ability to activate unsensitized T cells, thereby triggering a cellular antitumor immune response. Immature dendritic cells (imDCs) take up and process antigens. Mature dendritic cells (mDCs) then stimulate T cells that attack the tumor cells. They play a key role in transmitting information about antigens from peripheral tissues to areas of lymphoid tissue.
In the EU, more than 130 studies are currently being conducted using dendritic cells for various malignant neoplasms [4]. Doctors use this method for cervical tumors to reduce the risk of relapse after surgery or control cancer at an advanced stage for longer.
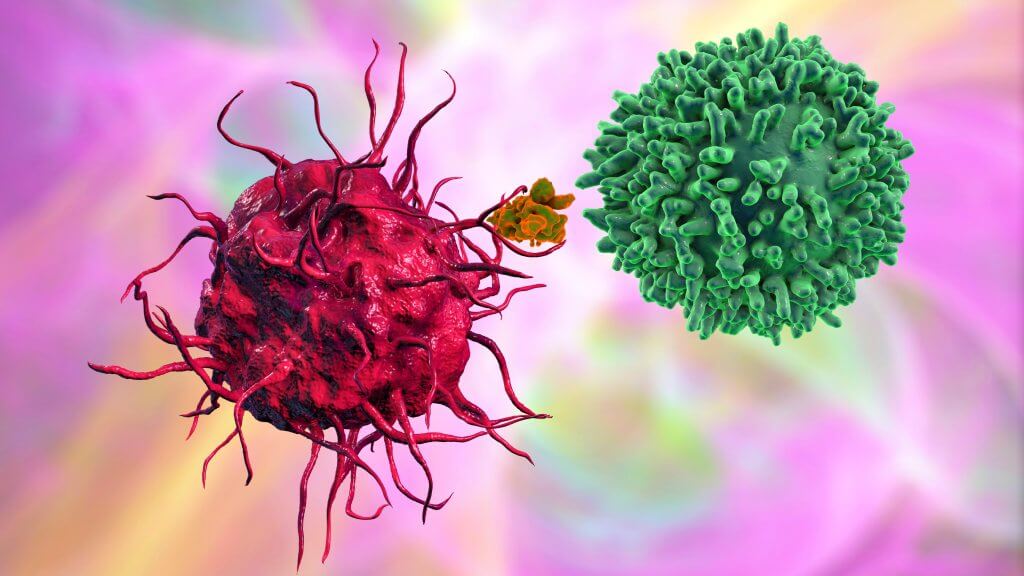
The role of the immune system and immunotherapy in cervical cancer treatment
Cervical cancer is the most common gynecologic cancer in the world [5]. Interestingly, this is one of the few oncological diseases against which one can be vaccinated. Almost all cases of cancer of the cervix are associated with the human papillomavirus [3]. Specific vaccines have been developed to protect against infection. However, in most countries in the world, total vaccination of the population is not carried out, and screening programs are poorly organized, so the incidence of cervical cancer remains very high. Almost half a million new cases are reported worldwide every year.
The standard treatment methods for the disease are surgery and radiation therapy. Cytostatic chemotherapy is also widely used. New directions of drug treatment, such as targeted therapy and immunotherapy, are still not widely used for malignant tumors in the cervix.
In standard treatment regimens, medications that influence the immune system can only be used in a limited number of patients if the tumor cells have increased PD-L1 protein expression. Only one PD-1 inhibitor is approved for clinical use, and it is applied as second-line therapy or for the suppression of recurrent tumors. The progress of effective cancer immunotherapy strategies is, however, ongoing – dendritic cell vaccines are being developed [6].
Dendritic cell therapy for early-stage cervical cancer
For patients with early-stage cervical tumors (stages IB and IIA), dendritic cell therapy offers hope for preventing cancer return and building long-term protection. This personalized treatment can be used alongside or after surgery to strengthen your body's natural defenses against cancer.
Even after successful surgery, cervical cancer can return in 20% of patients within five years when lymph nodes appear clear, rising to 50% when cancer has spread to distant lymph nodes. J Virol et al. suggested that the use of dendritic cells may reduce the risk of relapse, though the primary focus of early research was to establish safety and optimal dosing rather than measure survival outcomes [7].
Clinical studies have demonstrated several important benefits:
- Prevents recurrence by training other immune cells to recognize cancer permanently
- Creates personalized protection using dendritic cell vaccines made from your own blood immune cells
- Establishes lifelong activation of the immune system against specific cancer
- Works safely with no serious side effects reported in trials
- Integrates with surgery and other treatments
A University of Arkansas study followed 10 patients who received dendritic cell vaccines after radical surgery. The treatment involved injections over several weeks using mature autologous dendritic cells treated with human papillomavirus 16/18 E7 oncoprotein. All patients remained cancer-free during follow-up, demonstrating effective immune response without significant complications. Researchers found both cellular and humoral immune responses, confirming the dendritic cell vaccines' ability to generate a broad T cell immune system response and potentially destroy tumor cells with low disease burden. [7]
Dendritic cell therapy for end-stage cervical cancer
Advanced cervical cancer presents unique challenges, as patients often experience weakened immunity from previous treatments and face the physical burden of widespread disease. Despite these complexities, dendritic cell therapy offers meaningful hope by targeting all tumor sites throughout the body simultaneously, working to prevent further disease spread and halt the progression of metastases.
Unlike conventional treatments that may struggle to reach distant cancer sites, dendritic cell vaccines create a systemic immune response that can identify and attack cancer cells wherever they may be located. This comprehensive approach addresses not only existing tumors but also works to prevent new metastatic tumor growth, offering patients a chance to stabilize their condition and potentially extend survival.
The safety profile of dendritic cell therapy becomes particularly valuable for patients with advanced disease. Clinical studies demonstrate excellent tolerability and immune response with minimal side effects, making this treatment especially suitable for debilitated patients who may not tolerate aggressive conventional therapies. The treatment does not cause the severe complications often associated with intensive chemotherapy or radiation, allowing patients to maintain a better quality of life during treatment.
One of the most remarkable benefits documented in advanced cervical cancer patients is the significant pain relief achieved through dendritic cell vaccination. Research involving 20 patients with advanced T3-4N1M0-1 cervical cancer revealed that 95% of patients experienced significant immune response and complete pain relief after treatment sessions, allowing them to discontinue opioid medications entirely. This pain management effect represents a profound improvement in the quality of life for patients who previously required strong pain medications. [8]
The flexibility of dendritic cell therapy allows seamless integration with any existing treatment regimen. Patients can receive a dendritic cell vaccine alongside:
- Palliative chemotherapy or radiation therapy
- Targeted therapy medications
- Supportive care measures including pain management
- Nutritional support and rehabilitation services
- Other cancer immunotherapy approaches
This compatibility ensures that patients do not need to choose between dendritic cell therapy and other beneficial treatments. The dendritic cell vaccines can enhance the effectiveness of existing therapies while providing their own anti-cancer immune response.
While individual responses vary, the combination of safety, tolerability, and potential effectiveness makes dendritic cell therapy a valuable option for patients seeking comprehensive care for advanced cervical tumors.
| Therapy Type | 2-Year Survival Rate | Response Rate | Duration | Side Effects |
|---|---|---|---|---|
| Standard Treatments | ~25% for advanced cancer | Less than 10% | Several cycles | Severe (nausea, fatigue, hair loss, suppression of immune system, skin irritation) |
| Innovative Methods | ~60% for advanced cancer | 45-65% | Up to 4 sessions | Mild (localized discomfort) |
*Results vary based on individual patient factors, disease progression, and overall health status. Treatment outcomes should be discussed with qualified oncology specialists.
What is the process of cervical cancer treatment with DCs?
Treatment may differ from hospital to hospital, as dendritic cell immunotherapy for cervical cancer is not standardized yet. Thus, it is important to choose a specialized healthcare facility with vast practical experience in the field.
A general treatment regimen with dendritic cell vaccines looks as follows:
Dendritic cell harvesting. The process begins with taking 150-200 ml of blood from the patient, with the volume adjusted based on patient weight. Medical specialists then isolate immature progenitor cells from this blood sample for growing dendritic cells in the laboratory setting.
Dendritic cell processing. The laboratory cultivation takes place in certified facilities meeting EU-GMP requirements. Dendritic cells require 7 days to reach maturity during the laboratory growth process. Once mature, specialists evaluate the number of mature dendritic cells and their viability using a flow cytometer. The final step involves repeated cleaning of mature dendritic cells and removing non-viable cells to ensure treatment quality. This processing enables the dendritic cells to effectively train T cells and generate tumor specific T cells that can recognize and attack cancer cells.
Dendritic cell injection. Dendritic cells are administered through subcutaneous injection into the inguinal region or the area of lymphatic collectors close to the tumor, utilizing the natural lymphatic drainage pathways. This approach is combined with intravenous drip of vitamin preparations and symptomatic therapy to support overall patient care. The treatment stimulates a powerful immune response and activates T cell activity against cancer cells.
What results can be expected after cervical cancer treatment with DCs?
Dendritic cell therapy has demonstrated remarkable effectiveness across different stages of cervical cancer, with clinical evidence showing substantial improvements in patient outcomes. While individual responses vary based on the characteristics of cancer cells and overall health, the accumulated data from multiple medical centers reveals consistent patterns of success that offer genuine hope for patients facing this challenging disease.
The treatment shows particularly impressive results for advanced and metastatic cervical cancer, where conventional therapies often fall short. Clinical studies document response rates between 50-65%, representing a significant advancement over traditional approaches. These outcomes reflect not just temporary improvements but meaningful extensions in survival time, with many patients experiencing years of additional life with maintained quality.
One of the most compelling aspects of dendritic cell therapy lies in its dual benefit profile. Patients consistently report dramatic pain relief, with studies showing 95% of advanced cervical cancer patients achieving complete pain elimination after treatment completion. [8] This allows discontinuation of opioid medications while simultaneously fighting the cancer itself, addressing two critical aspects of advanced disease management.
| Stage of cancer | Standard treatments | Alternative medicine methods | Dendritic cell vaccination |
|---|---|---|---|
| Localized cancer | About 100% | Not used | Up to 90% |
| Locally advanced cancer | Up to 60–80% | Not used | Up to 90% |
| Metastatic cancer | Up to 20–40% | Up to 50% | Up to 70% |
*Booking Health data
Professor Frank Gansauge, a leading cancer immunotherapy specialist with over 22 years of experience in dendritic cell therapy, emphasizes that this treatment represents a proven therapeutic approach rather than an experimental option. His clinical practice has documented complete responses in cancer patients, with some achieving five-year disease-free survival. The safety profile remains exceptional, with minimal side effects reported across thousands of treatments worldwide.
The following interview with Professor Gansauge provides deeper insights into the clinical realities of dendritic cell therapy, addressing common concerns and explaining why this influencing the immune system approach has become an integral part of modern cancer care for patients seeking comprehensive treatment options.
Leading Immunotherapy Expert Prof. Frank Gansauge: "Revolutionizing Cancer Therapy with Dendritic Cell Vaccines"
Where to undergo dendritic cell therapy for cervical cancer?
Germany offers access to dendritic cell therapy for cervical cancer through specialized medical centers that have begun using this experimental treatment. While not yet standardized as a routine procedure everywhere, select German hospitals provide patients with this advanced immunotherapy option, particularly for those who have not responded to conventional treatments.
German medical centers specializing in this therapy offer several key advantages:
- State-of-the-art facilities for dendritic cell cultivation and processing with rigorous quality control
- Experienced oncologists who participate in international research and clinical trials
- Personalized treatment protocols tailored to each patient's specific tumor cells characteristics
- Integration with conventional therapies including surgery, chemotherapy, and radiation
- Stringent safety standards with comprehensive medical oversight
The Booking Health service connects patients with these German medical centers, providing transparent pricing and detailed information about available specialists. Our platform eliminates foreign patient taxes, making treatment more accessible while ensuring no unexpected costs through comprehensive insurance coverage.
A Medical Journey: Every Step of the Way With Booking Health
Navigating cervical cancer treatment decisions can feel overwhelming, especially when facing advanced disease or limited response to standard therapies. With different specialists offering varying opinions and treatment protocols that differ between medical centers, patients often struggle to identify the most suitable approach for their specific situation.
Booking Health has spent over 12 years connecting patients with innovative cervical cancer treatments, and developing expertise in complex gynecologic oncology cases. Our medical coordinators understand the nuances of dendritic cell therapy candidacy and the integration of immunotherapy with conventional treatments.
For patients considering cervical cancer treatment abroad, Booking Health provides comprehensive support:
- Medical report analysis and treatment program development
- Selection of suitable German medical centers with cervical cancer expertise
- Document preparation and clinic communication
- Pre-treatment consultations with specialists
- Continuous support during hospital stay and follow-up care
- Complete travel coordination including visas, accommodation, and transfers
- Personal coordinator with 24/7 multilingual support
- Transparent pricing with no hidden costs and insurance protection
When facing cervical cancer, your treatment decisions significantly impact both outcomes and quality of life. Partnering with experienced medical coordinators who understand advanced cervical cancer treatments ensures you receive personalized care tailored to your specific needs.
Dendritic Cell Therapy: Patient Stories with Booking Health
FAQ About Dendritic Cell Therapy for Cervical Cancer
Send request for treatmentDendritic cell therapy is a personalized immunotherapy that uses your own immune cells to create a cancer vaccine. The treatment trains your immune system to recognize and attack cervical cancer cells more effectively, offering hope for patients with both early and advanced stages of the disease.
Yes, dendritic cell therapy demonstrates excellent low toxicity profiles with minimal side effects. Unlike conventional treatments, patients experience no severe complications, making it particularly suitable for debilitated patients who may not tolerate aggressive therapies.
Dendritic cell therapy works alongside existing treatments rather than replacing them. It can be safely combined with chemotherapy, radiation, and other therapies to enhance overall treatment efficacy while providing additional anti-cancer benefits and improving quality of life.
Clinics in Germany offer dendritic cell vaccines through specialized medical centers. These facilities provide state-of-the-art cultivation and processing with experienced oncologists who participate in international research, ensuring comprehensive care for cervical cancer patients.
The treatment harvests your dendritic cells from blood, processes them with cancer antigens and HPV proteins, and then reinfuses them to stimulate a powerful T-cell response. This creates a lasting immune response that can identify and attack cancer cells throughout the body.
Eligible patients are those with early-stage disease seeking recurrence prevention, advanced cervical cancer cases, and metastatic cases where conventional treatments have limited effectiveness. The therapy is particularly valuable for patients seeking comprehensive treatment options with minimal side effects.
Benefits include tumor control, potential cancer regression, pain relief, prolonged survival, and the ability to maintain quality of life while fighting cancer. The treatment creates lifelong immunity against recurrent cancer.
Clinical studies show response rates of 50-65% in advanced cases, with up to 70% survival rates in metastatic disease. The treatment demonstrates consistent patterns of success across different stages, offering meaningful extensions in survival time with maintained quality of life.
The dendritic cell therapy price varies by medical center and treatment protocol. Germany treatment cost depends on individual case complexity and duration. Booking Health services provide transparent pricing with no hidden costs and comprehensive insurance coverage for international patients.
Several best cancer clinics in Germany and specialized immunotherapy centers provide this treatment. These facilities offer experienced oncologists, state-of-the-art processing capabilities, and comprehensive cervical cancer treatment in Germany.
Booking Health provides comprehensive medical travel for cervical cancer support including medical report analysis, clinic selection, document preparation, and development of a personalized treatment plan. Our coordinators offer 24/7 multilingual support throughout your treatment journey with the transparent cost of cervical cancer treatment information.
In patients with metastatic (stage 4) cervical cancer, dendritic cell vaccine shows a response rate of up to 70%, which is significantly higher than the effectiveness of standard methods (20-40%).
While standard treatment provides a 2-year survival rate of about 25%, innovative methods including dendritic cells increase this figure to 60%. They cause a mild local reaction instead of the severe complications characteristic of chemotherapy or radiation therapy.
The course of dendritic cell therapy is usually limited to 4 sessions while standard chemotherapy requires several cycles and lasts much longer. Despite the shorter course, the immune response remains active for a long time.
Dendritic cell vaccination has minimal side effects, usually mild local discomfort or redness at the injection site. Unlike standard methods the treatment does not cause nausea, hair loss or suppression of the immune system.
In the early stages (localized cancer), dendritic cell therapy shows up to 90% effectiveness, which is almost equivalent to the results of surgical treatment. It forms a long-lasting immune memory reducing the risk of relapse even after radical surgery.
In cases of locally advanced disease (stages II–III), dendritic cell therapy provides up to 90% efficacy, exceeding the results of standard regimens (60–80%). Through immune activation it enhances the effects of surgery or chemotherapy and improves tumor control.
In Germany cervical cancer patients receive dendritic cell therapy after thorough evaluation (especially tumor stage and immune profile). Treatments are personalized. In German hospitals treatments are carefully administered and continuously monitored (maintaining safety alongside standard oncology care).
Choose treatment abroad and you will for sure get the best results!
This article was edited by medical experts, board-certified doctors Dr. Nadezhda Ivanisova, and Dr. Bohdan Mykhalniuk. For the treatment of the conditions referred to in the article, you must consult a doctor; the information in the article is not intended for self-medication!
Our editorial policy, which details our commitment to accuracy and transparency, is available here. Click this link to review our policies.
Sources:
[1] WHO. Cervical cancer. https://www.who.int/news-room/fact-sheets/detail/cervical-cancer
[2] PubMed. Therapeutic vaccines for cervical cancer: dendritic cell-based immunotherapy. https://pubmed.ncbi.nlm.nih.gov/16248803/
[3] PubMed. Dendritic cell (DC) based therapy for cervical cancer: use of DC pulsed with tumour lysate and matured with a novel synthetic clinically non-toxic double stranded RNA analogue poly [I]:poly [C(12)U] (Ampligen R). https://pubmed.ncbi.nlm.nih.gov/12531360/
[4] EU Clinical Trials Register. Clinical trials for Dendritic cells. https://www.clinicaltrialsregister.eu/ctr-search/search?query=Dendritic+cells
[5] American Cancer Society. Cervical Cancer Leads Cancer Deaths for Women in 37 Countries. https://www.cancer.org/research/acs-research-news/cervical-cancer-leads-cancer-deaths-37-countries.html
[6] International Journal of Gynecological Cancer. 618 Hpv16/18 E7-Pulsed Dendritic Cell Vaccination in Patients With Recurrent Cervical Cancer Refractory to Standard Salvage Therapy. https://www.international-journal-of-gynecological-cancer.com/article/S1048-891X(24)05261-7/fulltext
[7] PubMed Central. Human Papillomavirus Type 16 and 18 E7-Pulsed Dendritic Cell Vaccination of Stage IB or IIA Cervical Cancer Patients: a Phase I Escalating-Dose Trial. https://pmc.ncbi.nlm.nih.gov/articles/PMC2258728/
[8] American Society of Clinical Oncology. Dendritic cell vaccine as an alternative to opioid analgesia in patients with advanced cervical cancer. https://meetings.asco.org/abstracts-presentations/186101
Read:
Cervical Cancer Treatments Guide
Article menu:
- What are dendritic cells?
- The role of the immune system and immunotherapy in cervical cancer treatment
- Dendritic cell therapy for early-stage cervical cancer
- Dendritic cell therapy for end-stage cervical cancer
- What is the process of cervical cancer treatment with DCs?
- What results can be expected after cervical cancer treatment with DCs?
- Where to undergo dendritic cell therapy for cervical cancer?
- A Medical Journey: Every Step of the Way With Booking Health
- FAQ About Dendritic Cell Therapy for Cervical Cancer
Don't know where to start?
Contact Booking Health