The second most common cancer diagnosis in men and the fifth cause of cancer deaths all over the world is prostate cancer. GLOBOCAN 2018 reports that the number of new cases was 1,276,106 cases worldwide, with the main increase in developed countries [1]. The mortality and the incidence are closely associated with age, and the rates are highest among men aged beyond 65 years. African-American patients are at the greatest risk and tend to get a more aggressive form of the disease than White men. With these differences, prostate cancer can be asymptomatic in its early form and is slow-growing, so in most cases, active surveillance is a possibility.
Detecting prostate cancer early is very important in enhancing survival. Early diagnosis not only can be used to manage the growth of cancer cells, but also to minimize the chances of complications like urinary incontinence and erectile dysfunction. This enables the patients to maintain their quality of life and enjoy modern and minimally invasive treatment options that are now offered in the most reputable clinics in Germany.
Surgery for Prostate Cancer as the Traditional Gold Standard
Over a long period of time, the treatment of prostate cancer depended mainly on surgery. The procedure known as radical prostatectomy was the most mainstream procedure to be performed on men with localized or advanced disease. Doctors hoped to avoid the spread of cancer cells because by removing the prostate along with the tumor, they could eliminate it. This surgical technique was the gold standard for decades, since other techniques of treating prostate cancer had not been developed yet to demonstrate similar survival rates.
Advantages of Radical Prostatectomy for Prostate Cancer
The idea of surgery is simple: remove the source of cancer and reduce the risk of recurrence. This was especially effective for younger patients with prostate cancer, who could tolerate major procedures and had a long life expectancy. Older patients, too, were frequently given surgery at increased risk. Over time, there have been technological improvements, including laparoscopic and robot-assisted surgery, which have become more successful. Currently, surgeons can carry out surgery on prostate cancer via smaller incisions, with less blood loss, and shorter recovery time.
Drawbacks and Side Effects of Surgery for Prostate Cancer
Surgery is a complicated process even today. The prostate is located near nerves and muscles that are in charge of the control of urination and sexual activity. Inevitably, these are often damaged during prostate cancer surgery, leading to long-term complications. The most common include urinary incontinence and erectile dysfunction. Even after minimally invasive operations, many men face a significant decline in quality of life. Furthermore, surgery does not guarantee the complete removal of microscopic cancer cells, so additional prostate cancer treatment, such as radiation or hormone therapy, may be required.
Psychological and Emotional Impact of Surgery for Prostate Cancer
Beyond the physical consequences, radical prostatectomy carries a psychological toll. The prostate is deeply linked to male identity and health. Losing it often affects self-esteem, intimate relationships, and overall emotional well-being. Research and patient testimonials show that the effects of surgery last for years. Many patients later admit they wish they had been offered less invasive prostate cancer therapy options before undergoing surgery.
Innovative Focal Treatment for Prostate Cancer
Over the past years, the treatment of prostate cancer has improved significantly beyond conventional surgery and radiation. Leading European clinics are currently actively practicing focal therapy of prostate cancer, an innovation that has proven to be one of the most important in the current oncology research. This procedure enables surgeons to selectively attack cancerous tumor foci and spares normal prostate tissue, unlike radical surgery, which removes the entire prostate. This would be especially useful with patients who seek a high quality of life and wish to minimize the side effects that occur over the long term, like urinary incontinence and erectile dysfunction.
The most advanced vascular targeted photodynamic (VTP) technology currently available is TOOKAD® VTP. This method uses a photosensitizing drug in combination with a targeted light source to destroy cancer cells within the prostate. Thanks to its minimally invasive nature, VTP therapy provides patients with a precise and effective treatment option for localized prostate cancer. Over the years, TOOKAD® VTP prostate cancer therapy has demonstrated consistent results, with minimal complications and high preservation of sexual and urinary function.
How TOOKAD® VTP Focal Therapy Works for Prostate Cancer
TOOKAD VTP has a specific mechanism that is unlike any other type of treatment for prostate cancer. A patient is first given an intravenous injection of a long-wavelength soluble vascular-targeted photodynamic drug that selectively attaches to blood vessels that supply the malignant tumor tissue. This compound does not affect healthy cells, unlike chemotherapy or radiation therapy, which also may affect healthy cells.
After the medication accumulates in the tumor vessels, a thin optical fiber is carefully inserted through a needle into the prostate under ultrasound or magnetic resonance imaging guidance. Once in position, the device emits laser light of a defined wavelength. This activates the photosensitizer, which causes a chemical reaction that forms free radicals. These molecules result in instant blood vessel shutdown to the tumor, interrupting oxygen and nutrients. The targeted cancer cells get killed quickly without having a blood supply, and the prostate tissue surrounding the affected area is also not affected.
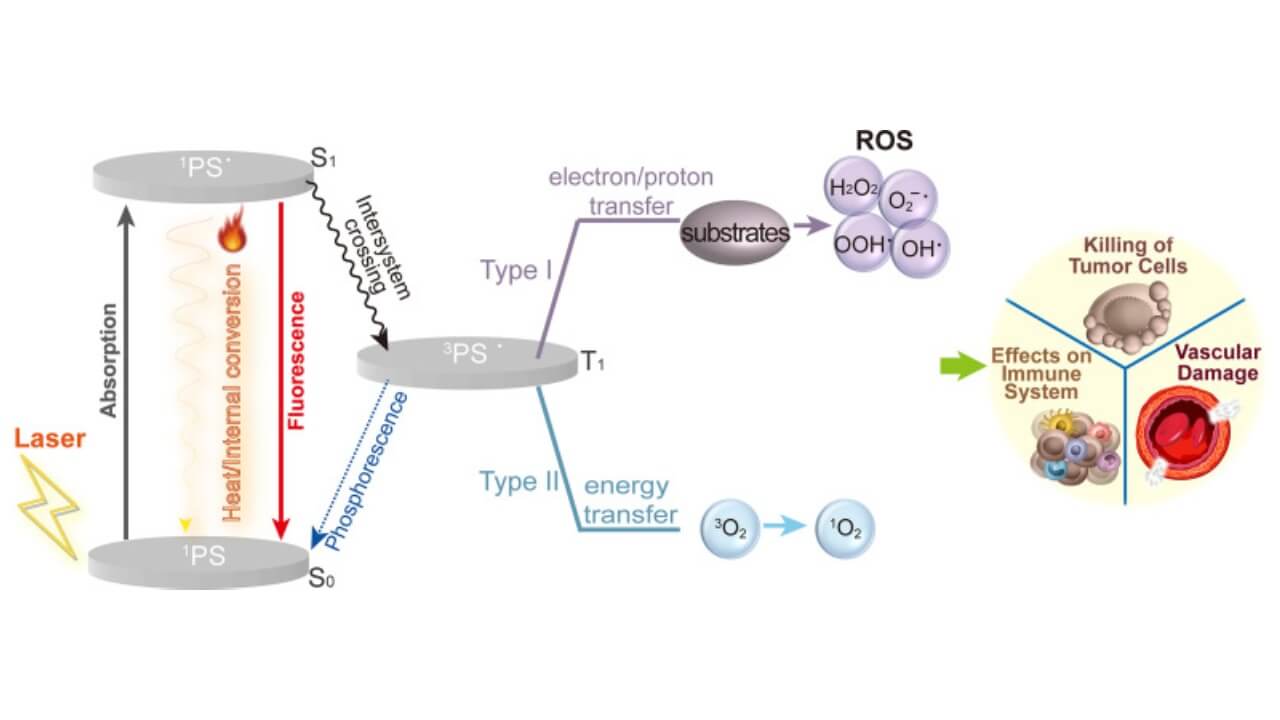
One of the main advantages of TOOKAD vascular targeted photodynamic therapy (VTP) is its precision. The light beam affects only the malignant tumor area, leaving healthy prostate tissue unharmed. Unlike traditional prostate cancer surgery, the prostate is not removed entirely, which significantly lowers the risk of urinary incontinence and erectile dysfunction. Because the organ and surrounding structures are preserved, patients experience faster recovery, minimal side effects, and better functional outcomes compared to standard prostate cancer therapy.
In practical terms, the entire TOOKAD VTP treatment typically lasts one to two hours, and patients can return home the same or the following day. Long-term results from European studies confirm that TOOKAD® VTP therapy provides effective control of localized prostate cancer while maintaining excellent quality of life. This combination of high precision, minimal invasiveness, and oncological effectiveness makes vascular targeted photodynamic therapy an advanced focal therapy for prostate cancer.
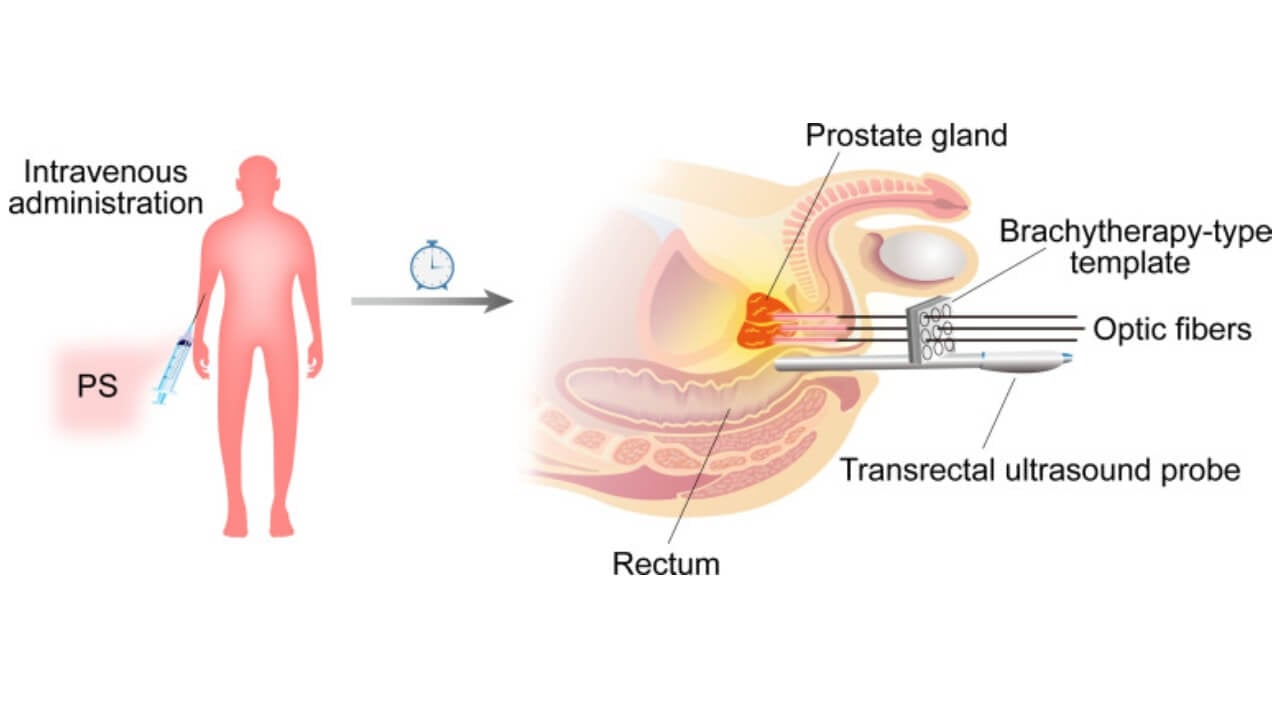
Patient Selection Criteria for TOOKAD® VTP for Prostate Cancer
TOOKAD VTP therapy is not suitable for all patients with prostate cancer. The best results are obtained through selectivity.
Typical criteria include:
- Localized prostate cancer, restricted to one lobe of the prostate
- Grades 6 or less: the tumor is low grade
- Foreseen life expectancy of over 10 years
- Clinical stage T1c or T2a, which will make sure that the disease is confined to the prostate gland
- PSA level ≤ 10 ng/mL
Proper patient selection enables patients undergoing TOOKAD VTP treatment to achieve long-term cancer control without excessive damage to surrounding tissues [3].
Advantages of TOOKAD® VTP for Prostate Cancer Over Conventional Treatments
Vascular targeted photodynamic (VTP) therapy represents a major shift in modern prostate cancer treatment. Compared to radical prostate cancer surgery, it is minimally invasive and preserves critical structures, which helps prevent urinary incontinence and erectile dysfunction. Patients experience fewer side effects, quicker recovery, and higher satisfaction with their quality of life.
TOOKAD VTP therapy can be repeated if new tumor foci appear. This is more applicable in the case of men who have low-risk prostate cancer and wish to use organ-saving treatment methods [4]. TOOKAD VTP treatment of prostate cancer targets only the involvement of diseased blood vessels and, in this manner, healthy prostate tissue and adjacent structures are maintained intact, making TOOKAD VTP therapy an effective and functional treatment.
The Future of TOOKAD® VTP for Prostate Cancer
Focal therapy has a good future in prostate cancer treatment. With magnetic resonance imaging, biopsy, and targeted photodynamic therapy, physicians are able to map the location of a tumor with precision and administer highly personalized treatment. The long-term outcome is that patients demonstrate good sexual function, urinary control, and overall quality of life, in addition to successful cancer control.
TOOKAD VTP therapy has since become a regular choice of European clinics to treat prostate cancer patients, particularly those with low-risk prostate cancer or low-grade disease. This initiative highlights a wider trend in oncology of more targeted, less invasive approaches, with a focus on disease management and patient welfare.
To learn more about the advanced approaches to prostate cancer treatment in Germany, including surgical options, targeted therapies, and personalized care plans, we recommend watching an interview with Dr. Birte Schneevoigt. She shares insights on how German urologists manage prostate cancer at various stages and what international patients can expect when seeking treatment.
Combination of TOOKAD® with Other Prostate Cancer Treatments
Flexibility is one of the advantages of the vascular targeted photodynamic (VTP) therapy with TOOKAD. Although it may be conducted as a focal therapy alone treatment in men with low-risk prostate cancer, current studies have demonstrated encouraging effects when VTP therapy is used alongside other well-proven therapies. This integrated strategy will provide fresh insights to patients with prostate cancer and those who want to undergo minimally invasive procedures that optimize tumor management and quality of life.
VTP TOOKAD® and Active Surveillance in Prostate Cancer
In men who have low-grade disease but first opt to use active surveillance, VTP treatment may be an intermediate measure:
- It assists in the stabilization of the disease and minimization of its progression
- TOOKAD is used in many patients who do not necessarily proceed to radical surgery or radiation treatment
- Prostate tissue sparing lowers the chances of side effects, including erectile dysfunction or impaired urinary flow.
This combination enables patients with prostate cancer to further receive close monitoring and, at the same time, enjoy the benefits of the targeted destruction of cancer cells.
VTP TOOKAD Therapy Combined with Surgery in Prostate Cancer
Vascular targeted photodynamic therapy may be used in combination with surgery in cases of local prostate cancer:
- Preoperative: reducing the size of the tumor will make the process less aggressive and more accurate
- Postoperative: use of focused photodynamic VTP therapy to eliminate remaining cancer cells in the prostate bed reduces the chances of recurrence
Though currently under investigation, this multimodal approach is promising to yield better results in selected patients.
Integration with Radiation Therapy in Prostate Cancer
Photodynamic therapy has also been explored in conjunction with external beam radiation. Benefits include:
- Reduction of tumor volume before radiation
- Possibility to decrease total radiation dose, minimizing side effects
- Enhanced destruction of resistant cells within the prostate tissue
Preliminary studies demonstrate that using VTP TOOKAD in this way may improve overall effectiveness without sacrificing quality of life.
Combination with Systemic Therapies in Prostate Cancer
At high risk or advanced disease, researchers are testing a combination of soluble vascular targeted photodynamic therapy with systemic therapies, including:
- Hormone therapy is administered to prevent the growth of the cancer
- New drugs that attack the cancer cells that circulate outside the prostate
This is a dual approach that combats the local and systemic components of the disease, and the signs of success are shown in patients receiving the treatment.
Long-Term Outlook
Making VTP TOOKAD prostate cancer therapy a part of a multimodal care provides:
- Greater control of the tumor
- Maintenance of urinary and sexual functioning
- Better quality of life and long-term outcome among patients with prostate cancer
The role of targeted photodynamic therapy VTP in terms of prostate cancer management should be established in future studies with the help of magnetic resonance imaging follow-up.
Long-Term Follow-Up After VTP TOOKAD Therapy in Prostate Cancer
Vascular targeted photodynamic therapy (VTP) using TOOKAD is a highly effective and minimally invasive treatment method for localized prostate cancer, but it is essential that proper follow-up is used to achieve stable long-term outcomes. The recovery process is not limited to the hospital stay; regular monitoring allows doctors to assess the condition of the prostate tissue, detect possible recurrence, and confirm that cancer cells have been completely destroyed. German clinics provide structured follow-up programs designed for both local and international patients.
PSA Testing and Imaging Control in Prostate Cancer
After VTP treatment, the prostate-specific antigen (PSA) level becomes the primary marker for tracking disease activity. Patients who are undergoing the TOOKAD prostate cancer therapy normally do the PSA tests after every three months in the first year and then after every six months. Besides, magnetic resonance imaging is often prescribed to assess the internal prostate structure. These combined measures help confirm that the procedure has achieved durable control of the disease.
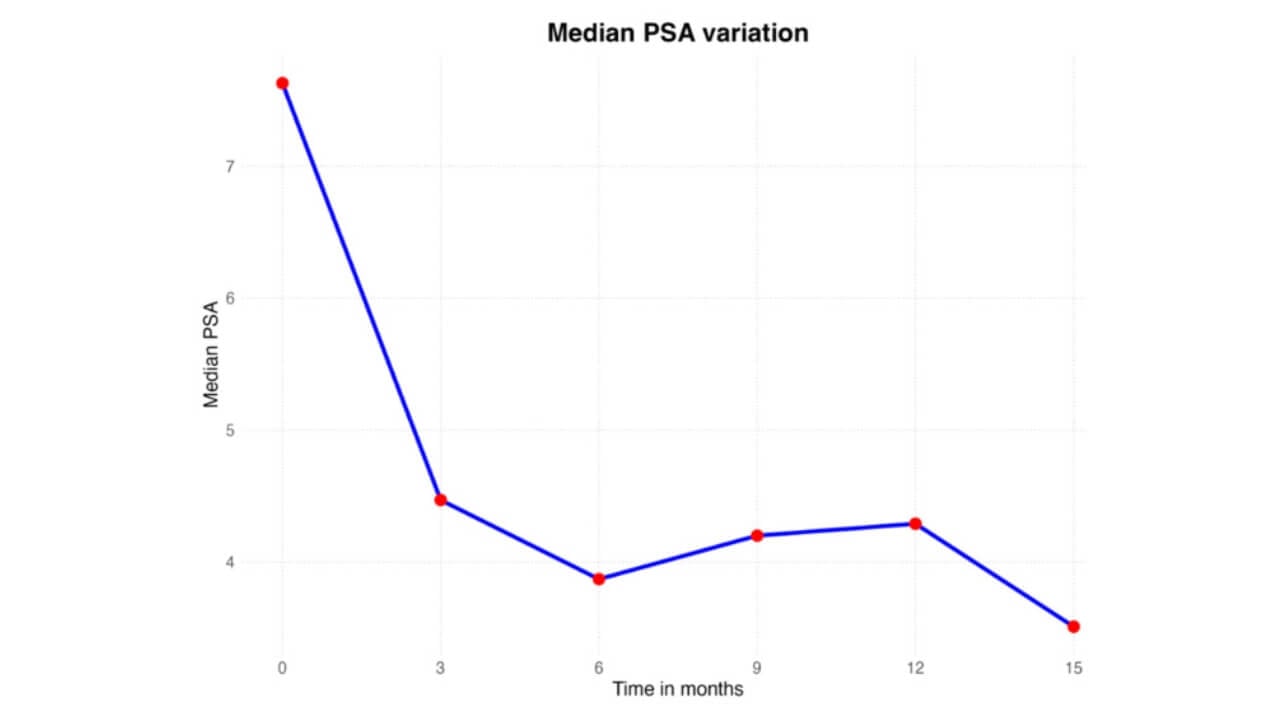
Biopsies and Pathology Reports in Prostate Cancer
In some cases, doctors recommend repeat biopsies to confirm the absence of active cancer cells. Unlike radical surgery, where the gland is removed, focal therapy requires close observation of the preserved prostate tissue. These biopsies provide precise information on whether the treatment remains effective in the long term.
Quality of Life After Treatment of Prostate Cancer
One of the main reasons men with low-risk or low-grade prostate cancer choose targeted photodynamic VTP therapy is its ability to maintain a good quality of life. Long-term studies in Germany confirm that the majority of patients with prostate cancer continue to enjoy normal urinary and sexual function. Rates of erectile dysfunction and urinary incontinence remain significantly lower compared to traditional surgery or radiation. This means that patients not only benefit from oncological safety but also preserve daily comfort and well-being.
Preventing Recurrence of Prostate Cancer
Ongoing monitoring is essential to detect possible regrowth of tumor tissue. If recurrence occurs, it is usually identified at an early stage, when additional treatment options, including repeat VTP therapy or alternative photodynamic therapy, are still possible. This flexibility ensures that patients remain under control without compromising their long-term results.
Why Do Patients Choose Germany for Prostate Cancer Treatment?
Germany has established itself as a global leader in prostate cancer treatment, especially in the field of focal therapy for prostate cancer. For patients seeking the most effective, minimally invasive, and targeted solutions, German clinics provide a combination of cutting-edge technology, extensive clinical experience, and internationally recognized standards. Germany was the first to formally endorse clinical guidelines to use TOOKAD vascular targeted photodynamic (VTP) on localized prostate cancer in 2022. This historic measure highlights how the nation is determined to provide safe, effective, and standardized prostate cancer treatment.
In the past, focal treatment procedures were too varied, and prospective controlled trials were lacking to make the creation of international guidelines easier. Germany has been a pioneer in overcoming these obstacles, offering clear, evidence-based recommendations that benefit both doctors and patients. These guidelines cover precise patient selection, imaging requirements such as magnetic resonance imaging, procedural standards, and long-term follow-up to monitor cancer cells and prevent recurrence.
Comprehensive Clinical Guidelines for Prostate Cancer
The latest update of the German guidelines for prostate cancer treatment includes a dedicated section on focal photodynamic therapy. It includes 12 statements and recommendations that are specifically related to the use of TOOKAD VTP therapy in treating patients with prostate cancer, with special attention to the localization of the tumor, patient selection, and long-term follow-up.
Based on these recommendations, TOOKAD VTP treatment is advised in men with low-risk prostate cancer or in low-grade tumors, in which the tumor is limited to a single lobe of the prostate. The guidelines also include steps to be used to ensure minimal destruction of neighboring prostate tissue, which maintains urinary and sexual function. With these tips, the success rates of German clinics are very high, and fewer side effects, less erectile dysfunction, and a high quality of life are all experienced by patients who are treated.
Vascular targeted photodynamic therapy in Germany is not an experimental technology but a tested, safe, and effective technique for treating prostate cancer.
Advanced Technology and Expertise in Prostate Cancer
The fact that medical teams have extensive experience in performing the TOOKAD VTP procedure is one of the main reasons why patients prefer to go to Germany when treating prostate cancer. German hospitals have conducted thousands of procedures, which provides the doctors with unmatched knowledge in patient selection, performing the procedure, and subsequent care.
The high precision is achieved by combining the vascular targeted photodynamic therapy with the sophisticated imaging technologies like magnetic resonance imaging. The advantage of this approach is that the patients experience the destruction of cancer cells and the maintenance of healthy prostate tissue. This minimizes the chances of developing long-term complications like erectile dysfunction and urinary incontinence, common after radical prostate cancer surgery.
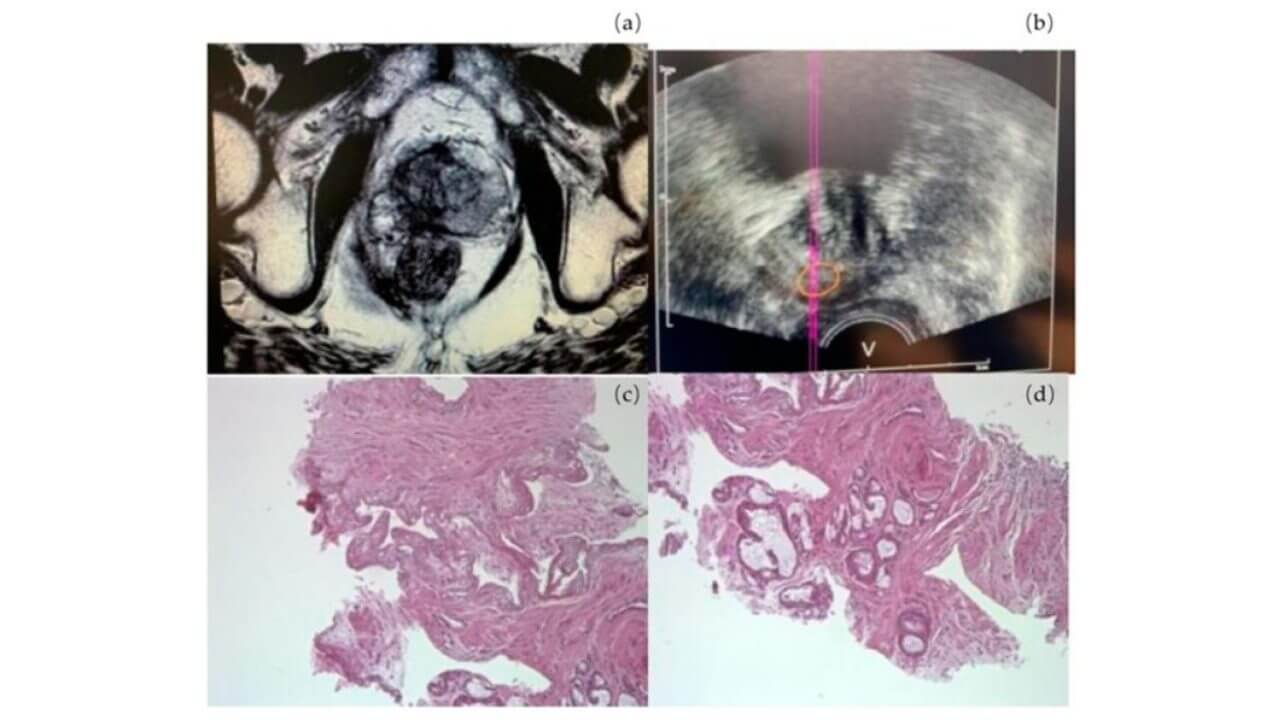
a) Fusion biopsy
b) Tumor area GS 6
c) Area of necrosis
d) The line and circle represent a machine function during a biopsy
Moreover, German clinics are equipped with the latest diagnostic and monitoring tools, ensuring that each patient receives highly personalized care. From pre-treatment imaging to follow-up assessments, doctors can measure the effectiveness of VTP therapy and adjust strategies for optimal outcomes. This results in superior long-term results and a higher quality of life compared to conventional prostate cancer therapy.
Comfortable Prostate Cancer Patients Experience and International Accessibility
Germany does not only boast of medical expertise, but also offers comfortable and patient-friendly environments. The major hospitals are equipped with advanced infrastructure and customized services, and thus international patients find it less difficult to have prostate cancer treatment.
The choice to undergo less invasive TOOKAD VTP treatment under a supportive and familiar environment lowers anxiety, boosts recovery, and improves the overall quality of life. Clinics make sure that the patient treated has an understanding of each stage, from initial consultation to long-term follow-up, reduce side effects, and do not lose sexual and urinary functions.
Further, medical tourism providers have streamlined procedures for international patients, such as travel arrangements, booking appointments, and insurance support. This is the reason behind the high number of patients who prefer German clinics to treat them with TOOKAD VTP and other advanced treatments for prostate cancer.
Proven Long-Term Prostate Cancer Treatment Effectiveness
Clinical trials in Germany reveal that TOOKAD VTP vascular-targeted photodynamic therapy has produced the best long-term outcomes. Subsequent data indicate that tumors are controlled, recurrence is minimal, and sexual and urinary functions are preserved. In comparison to radical surgery, patients treated with TOOKAD VTP report much lower rates of erectile dysfunction (and other side effects).
German clinics maximize the effect of treatment and reduce the harm by targeting cancerous cells with high accuracy and avoiding normal prostate tissue. This is an effective alternative to radical prostate cancer surgery, especially in men who have low-risk prostate cancer or lesions of low grade.
The integration of advanced technology and standardized protocols with a long history of clinical practice has made Germany a top choice for patients seeking innovative, minimally invasive treatment of prostate cancer with great outcomes in the long term.
VTP Prostate Cancer Cure in Germany
The price of the innovative focal therapy procedure treatment in Germany depends on several factors, such as:
- The complexity of the case of the patient
- The stage of the disease
- The details of the personal treatment plan
- The duration of the hospital stay
Moreover, the price can also depend on the reputation and location of the hospital.
Among the best hospitals offering this procedure are:
- Hospital Nuremberg
- Asklepios Hospital Hamburg
- University Hospital Munich
- Hospital Nordwest Frankfurt
- Helios Hospital Berlin
All these health facilities have state-of-the-art equipment and offer high-quality medical services.
| Type of treatment / Characteristics | Radical Prostatectomy | VTP TOOKAD® for prostate cancer treatment |
|---|---|---|
| Complications rate (impotence and urinary incontinence) | Up to 55% experience urinary incontinence, approximately up to 75% report erectile dysfunction of different severity | Less than 6% experience urinary incontinence, less than 15% have erectile dysfunction |
| Treatment duration | One-time procedure, duration of the intervention depends on the techniques used | One-time procedure typically lasting 90 minutes |
| 5-year survival rate | Up to 80% for early stages | Up to 95 % |
| Cost, € | €25,700 - €48,500 | €10,000 - €25,000 |
*Depends on the technique used.
**Depends on the stage of the disease and surgical approach used.
TOOKAD® treatment is performed only by doctors who have completed specialized training in vascular-targeted photodynamic therapy procedures. This ensures high expertise in using TOOKAD®.
A Medical Journey: Every Step of the Way With Booking Health
Finding the best treatment strategy for your clinical situation is a challenging task. Being already exhausted from multiple treatment sessions, having consulted numerous specialists, and having tried various therapeutic interventions, you may be lost in all the information given by the doctors. In such a situation, it is easy to choose a first-hand option or to follow standardized therapeutic protocols with a long list of adverse effects instead of selecting highly specialized innovative treatment options.
To make an informed choice and get a personalized cancer management plan, which will be tailored to your specific clinical situation, consult medical experts at Booking Health. Being at the forefront of offering the latest medical innovations for already 12 years, Booking Health possesses solid expertise in creating complex management programs in each individual case. As a reputable company, Booking Health offers personalized treatment plans for prostate cancer with direct clinic booking and full support at every stage, from organizational processes to assistance during treatment. We provide:
- Assessment and analysis of medical reports
- Development of the medical care program
- Selection of a suitable treatment location
- Preparation of medical documents and forwarding to a suitable clinic
- Preparatory consultations with clinicians for the development of medical care programs
- Expert advice during the hospital stay
- Follow-up care after the patient returns to their native country after completing the medical care program
- Taking care of formalities as part of the preparation for the medical care program
- Coordination and organization of the patient's stay in a foreign country
- Assistance with visas and tickets
- A personal coordinator and interpreter with 24/7 support
- Transparent budgeting with no hidden costs
Health is an invaluable aspect of our lives. Delegating management of something so fragile yet precious should be done only to experts with proven experience and a reputation. Booking Health is a trustworthy partner who assists you in pursuing stronger health and a better quality of life. Contact our medical consultant to learn more about the possibilities of personalized treatment with innovative methods for prostate cancer with leading specialists in this field.
Modern Cancer Treatment: Patient Journeys with Booking Health
FAQ: VTP TOOKAD Therapy for Prostate Cancer
Send request for treatmentTOOKAD soluble vascular-targeted photodynamic therapy (VTP therapy) is a new, minimally invasive approach to the treatment of prostate cancer, especially localized prostate cancer. The process involves the destruction of only those segments of the prostate that have cancer cells and leaving out the healthy prostate tissue. Patients enjoy diminished side effects, reduced erectile dysfunction, and improved quality of life. Treatment of VTP is particularly appropriate in men with low-grade lesions and low-risk prostate cancer, and it has proven to offer effective treatment with good survival outcomes.
Vascular targeted photodynamic therapy (VTP) commences with the precise localization of the tumor by imaging and biopsy. A soluble vascular targeted photodynamic drug is injected intravenously under anesthesia. Laser fibers are then used to trigger the photosensitizer at a particular wavelength, killing the cancerous cells and sparing the normal prostate tissue. It is a low-invasive procedure, requires 1-2 hours, and does not affect urinary function or sexual health, which provides patients with a high probability of a good quality of life after treatment.
This VTP treatment is advised for adult patients who have localized prostate cancer that is limited to one lobe. It must have low-grade disease, PSA ≤ 10 ng/mL, and no previous treatment of prostate cancer in most cases. German guidelines focus on proper patient selection so that the maximum benefit is achieved. This is among the most effective prostate cancer treatments because patients who have been treated according to these criteria have high long-term outcomes and minimal side effects.
TOOKAD(r) VTP treatment has several benefits as compared to radical prostate cancer surgery or standard radiation. It is the least invasive, it requires a short recovery time, and it does not leave behind healthy prostate tissue. The risk of urinary incontinence and erectile dysfunction is much less. In men with low-risk prostate cancer, studies indicate that this vascular targeted photodynamic therapy is an effective way of destroying local tumors with excellent long-term outcomes that are similar to other more invasive procedures.
The treatment is less likely to cause side effects compared to conventional treatments. Temporal problems can be urinary retention, slight incontinence, or erection problems. The activated soluble vascular targeted photodynamic drug can cause photosensitivity, and the patients should avoid exposing their bodies to light for 48 hours. German clinics monitor patients closely.
TOOKAD prostate cancer recovery is of short duration. The patients remain on medical care during the initial 6 hours, should not be exposed to sunlight during the 12-48 hours and should wear protective clothing and glasses. VTP therapy is done using minimally invasive procedures that enable the majority of patients to go home on the same day. Maintenance of healthy prostate tissue reduces loss of erectile and urinary complications.
Clinical trials indicate that out of every 50 patients with low-risk prostate cancer a TOOKAD VTP treatment, 50 percent of patients had no cancer cells detected in their follow-up biopsies two years later. Such a vascular targeted photodynamic therapy has high local control of the tumor, spares normal prostate tissue, and reduces side effects like erectile dysfunction. The results of the long-term follow-up indicate that the procedure is effective, safe, and promotes the quality of life, which is why it is a highly recommended choice in men with low-risk prostate cancer in need of minimally invasive treatment.
Choose treatment abroad and you will for sure get the best results!
Authors:
The article was edited by medical experts, board certified doctors Dr. Nadezhda Ivanisova and Dr. Bohdan Mykhalniuk. For the treatment of the conditions referred to in the article, you must consult a doctor; the information in the article is not intended for self-medication!
Our editorial policy, which details our commitment to accuracy and transparency, is available here. Click this link to review our policies.
Sources:
[1] Prashanth Rawla. Epidemiology of Prostate Cancer. World J Oncol. 2019 Apr 20;10(2):63–89. doi: 10.14740/wjon1191. [DOI] [PMC free article]
[2] Qin Xue, Jingliang Zhang, Jianhua Jiao, Weijun Qin, Xiaojian Yang. Photodynamic therapy for prostate cancer: Recent advances, challenges and opportunities. Front Oncol. 2022 Sep 23;12:980239. doi: 10.3389/fonc.2022.980239. [DOI] [PMC free article]
[3] Abdel-Rahmène Azzouzi, Eric Barret, Caroline M Moore et al. TOOKAD(®) Soluble vascular-targeted photodynamic (VTP) therapy: determination of optimal treatment conditions and assessment of effects in patients with localised prostate cancer. BJU Int. 2013 Oct;112(6):766-74. doi: 10.1111/bju.12265. [DOI] [PubMed]
[4] Abdel-Rahmene Azzouzi, Souhil Lebdai, Fawzi Benzaghou, Christian Stief. Vascular-targeted photodynamic therapy with TOOKAD® Soluble in localized prostate cancer: standardization of the procedure. World J Urol. 2015 Mar 19;33(7):937–944. doi: 10.1007/s00345-015-1535-2. [DOI] [PMC free article]
[5] Pietro Saldutto, Fernando Cavacece, Roberto La Rocca et al. The Safety and Efficacy of Vascular-Targeted Photodynamic Therapy in Low-Risk Prostate Cancer. Cancers (Basel). 2025 Feb 16;17(4):661. doi: 10.3390/cancers17040661. [DOI] [PMC free article]
Read:
A Comprehensive Guide to Getting Prostate Cancer Treatment
Immunotherapy for prostate cancer in Germany
Article menu:
- Surgery for Prostate Cancer as the Traditional Gold Standard
- Innovative Focal Treatment for Prostate Cancer
- Combination of TOOKAD® with Other Prostate Cancer Treatments
- Long-Term Follow-Up After VTP TOOKAD Therapy in Prostate Cancer
- Why Do Patients Choose Germany for Prostate Cancer Treatment?
- A Medical Journey: Every Step of the Way With Booking Health
- FAQ: VTP TOOKAD Therapy for Prostate Cancer
Don't know where to start?
Contact Booking Health