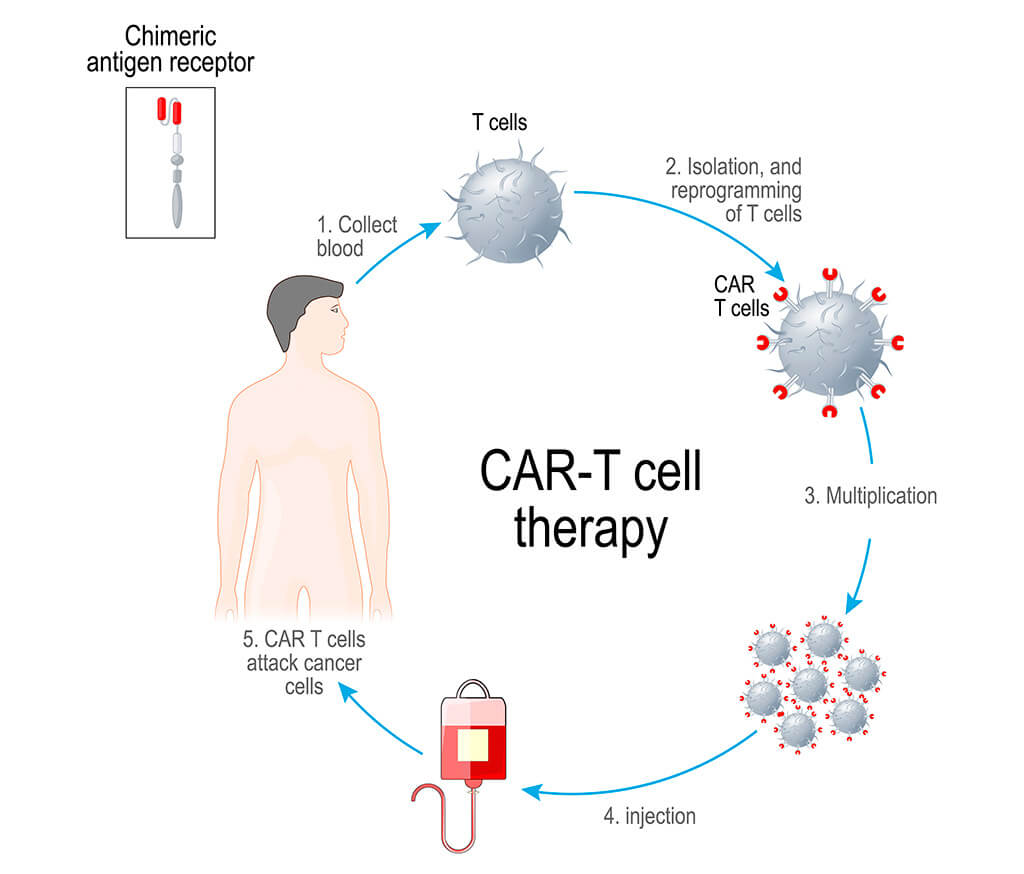
The American Society of Clinical Oncology publishes a Clinical Cancer Advances report annually. CAR T-cell therapy was presented as one of the main achievements of the year in the report for 2018. This therapy was a breakthrough in oncology, mainly in the treatment of hematologic diseases. Today, some countries use CAR T immunotherapy not only in clinical trials, but also in practical medicine. If you need information about the best specialized hospitals and prices for CAR T-cell immunotherapy, you can easily find it on the Booking Health website.
Content
- What is a chimeric antigen receptor?
- Why does CAR only destroy tumor cells?
- CAR production stages
- What types of cancer can be treated with CAR T-cell therapy?
- Can any side effects occur?
- Which clinical trials confirm the effectiveness of CAR therapy?
- The future of CAR therapy
- How can I undergo CAR therapy?
What is a chimeric antigen receptor?
The chimeric antigen receptor (CAR) is a recombinant receptor, which enables a CD-8 T-lymphocyte (immune cell) to activate and interact with a tumor cell. The CAR molecule has a complex structure. It consists of several parts, such as:
1. Target domain is used for the detection and recognition of cancer cells. It should match the tumor cell, just as a key fits the lock. If the "key" is absent, then the immune cell does not see the tumor cell, and therefore cannot destroy it.
2. Transmembrane domain serves for the fixation of the chimeric receptor on the surface of an immune cell.
3. Flexible hinge region improves target identification and provides receptor mobility.
4. Intracellular transmembrane domain, which is located inside the immune cell, on its membrane. It provides direct destruction of a malignant cell. This structure consists of the following two parts:
- Activating signal domain causes the self-destruction of a cancer cell and facilitates the multiplication of lymphocytes (immune cells)
- Co-stimulating domain activates lymphocytes and increases their life expectancy
During the use of CAR in oncology, several generations of chimeric antigen receptors will have already appeared. The co-stimulating domain is present in the second generation of CAR. There may be several such domains in the next, third generation.
Why does CAR only destroy tumor cells?
If CAR destroуed all cells in our body, the treatment would not be possible. These receptors work on the principle of a "homing missile". They are injected into the body, where they independently find and destroy malignant foci. At the same time, CAR T immunotherapy does not harm healthy tissues.
The targets for CAR are antigens that are absent in healthy cells. These antigens are only present on the surface of cancer cells. Therefore, CAR therapy is well tolerated by most patients. You should not be afraid that excessively active immunity will destroy your body.
CAR production stages
The treatment includes several stages:
- Harvesting leukocytes. Blood is drawn from a patient. It is centrifuged in order to obtain leukocytes. The doctors need at least 100 million immune cells. Blood sampling is carried out prior to chemotherapy starting. This is due to the fact that chemotherapy inhibits the hematopoietic function of red bone marrow. As a result, the doctors will need to take much more blood to harvest the required amount of leukocytes. The obtained material can be either frozen or used immediately for the CAR synthesis. Freezing has both advantages and disadvantages. On the one hand, it allows a doctor to choose the best time for the treatment, on the other hand, the number of cells decreases slightly.
- Activation of leukocytes. The magnetic carrier anti-CD3/CD28 antibodies are most often used for activation, but other methods are also applied. Autologous (own) or artificial antigen-presenting cells can be used for the activation of leukocytes.
- Genetic modification of cells. Lentiviral and gammaretroviral vectors are used. Another option is to use transposon/transposase systems ("Sleeping beauty" or "Piggyback").
- Multiplication of modified cells. This process takes place in bioreactors and involves cytokines (interleukin-2, IL-7, IL-15). GE WAVE bioreactors are most commonly used. They mix cells, increase their number, and maintain temperature and gas composition of the medium.
- Cell testing is carried out before their injection into the patient's body. The functional activity of cells, their microbiological safety, and transduction efficiency are examined.
Thus, the doctors receive an individual, self-reproducing drug, which is then injected into the patient's body.
What types of cancer can be treated with CAR T-cell therapy?
The first successful treatment results of CAR therapy were obtained in a neuroblastoma. In 2008, the use of first-generation CAR T-cells made it possible to achieve complete remission in 11 patients with this disease. Since then, the method has been tested for various types of cancer.
Most often, CAR is used in the treatment of lymphomas, leukemia, and myeloma. The greatest progress has been made in this particular medical field.
CAR T-cell immunotherapy demonstrates good results in the following types of cancer:
- Non-small cell lung cancer
- Melanoma
- Prostate cancer
- Kidney cancer
- Glioma
- Osteosarcoma
- Ovarian cancer
- Peritoneal cancers
- Gastrointestinal stromal tumors
- Mesothelioma
- Cervical cancer
Can any side effects occur?
CAR therapy can cause side effects and complications. It can reduce the number of B-lymphocytes. Some patients experience neurotoxicity or cytokine storm syndrome (very severe inflammation).
A lack of B-lymphocytes is the most common side effect, but it is a mild one. B-lymphopenia is dangerous because it leads to a deficiency of gamma globulins, but they can be injected into the patient intravenously, so the deficiency is compensated for. Upon the completion of CAR therapy, the normal number of B-lymphocytes is restored.
A cytokine storm syndrome manifests itself with an increased body temperature, lower blood pressure, and impaired function of internal organs.
Which clinical trials confirm the effectiveness of CAR therapy?
Dozens of successful CAR T therapy trials have already been carried out around the world. Some of their results are given below:
- The JULIET trial was carried out with the participation of 81 patients with a diagnosis of relapsed or refractory diffuse large B-cell lymphoma. About 53% of patients responded to the treatment. Almost 40% of them had a complete response. In 90% of these patients, a complete response was maintained after 6 months of monitoring. Overall relapse-free survival after 6 months was 73% in the complete response group and 61.5% in the partial response group.
- ZUMA trial. Patients with different types of B-cell lymphoma were examined. The overall response rate was 82%. In 58% of patients, the response was complete, and in 24% – partial. In 40% of patients, after 15 months of follow-up monitoring, the state of complete remission was maintained. The overall 18 month patient survival rate was 52%.
- KYMRIAH trial. The doctors received similar results. The overall response rate has reached 81%. The complete response was in 60% of patients. The overall survival rate of patients for 6 and 12 months was 90% and 76%, respectively. Relapse-free survival rates for the same follow-up periods were 73% and 50%.
Some people may find the results of such treatment rather modest. Indeed, the very expensive CAR therapy does not guarantee the patient a cure for cancer, but do not forget that the treatment is used for the most aggressive tumors. Prior to CAR therapy, this category of patients had almost no chance to defeat cancer. This treatment method increases the chance of remission by 10 times, in comparison with the previously used therapies. It also reduced the risk of death from onco-hematological diseases by 70%.
The future of CAR therapy
Currently, CAR therapy has many unresolved issues. The drugs which are used in clinical practice are monospecific. They are aimed only at certain target antigens, but if the tumor changes, the treatment becomes ineffective.
Other problems:
- high risk of cytokine storm;
- insufficient effectiveness against solid (dense) tumors.
Scientists around the world are constantly working in order to improve CAR therapy. A universal system (UniCAR) is being developed, which could target multiple targets at once, and also change them after the beginning of the therapy. If necessary, CAR must also be able to "turn off", which helps prevent severe complications from treatment.
The research on a number of systems which will improve the success rates and safety of CAR T-cell immunotherapy, have already been carried out. An example is SUPRA CAR. This is a modular CAR system, which uses the leucine zipper motif. SUPRA means "split, universal, and programmable". It allows doctors to select different targets on cancer cells without additional manipulations with the patient's immune cells. A unique property of SUPRA CAR is the ability to configure many parameters that can regulate the response of T-cells, including the prevention of their excessive activation, if necessary.
Another issue is price. The high cost of CAR T-cell therapy is related to the expensive, high-tech laboratory process of T-lymphocyte modification and cultivation. At the same time, the modern equipment of German clinics and the high level of qualification of specialists make treatment in Germany 30-50% cheaper than in US medical centers.
How can I undergo CAR therapy?
You can use the services of the Booking Health medical tourism operator. We will fully arrange your treatment in Germany or another country for you. The range of Booking Health services include:
- Selection of the best clinic which carries out CAR therapy with good results and a low risk of complications
- Reduced waiting for a treatment, which is especially important for rapidly progressing hematologic diseases
- Reduced prices for medical services; costs are reduced due to the absence of overpricing for foreign patients
- Direct communication with the doctor who carries out CAR therapy
- Elaboration of a treatment program without repeating previously performed examinations
- Buying and forwarding of medicines
- Communication with the doctor and hospital after the treatment program is complete
- Organization of additional examinations, treatment, or rehabilitation
Booking Health also provides top-class organizational services. We will book an apartment and airline tickets for you, arrange your transfer from the airport, and provide an interpreter and personal medical coordinator.
Choose treatment abroad and you will for sure get the best results!
Authors:
This article was edited by medical experts, board-certified doctors Dr. Nadezhda Ivanisova, and Dr. Bohdan Mykhalniuk. For the treatment of the conditions referred to in the article, you must consult a doctor; the information in the article is not intended for self-medication!
Our editorial policy, which details our commitment to accuracy and transparency, is available here. Click this link to review our policies.
Sources:
National Center for Biotechnology
Read:
New Effective Treatments for Stage 4 Cancer: Innovations in Oncology
Top 10 Leading Oncology Hospitals for Cancer Treatment in Germany
Don't know where to start?
Contact Booking Health