Prostate cancer ranks as one of the most widespread cancer types in men across the globe and continues to be a major reason for cancer-related deaths. The Global Cancer Observatory (GLOBOCAN) reports that doctors diagnosed over 1.4 million new cases of prostate cancer in 2020, making it the second most common cancer in men after lung cancer [1]. Even with better early detection methods, many men still present with advanced prostate cancer. This stage is harder to treat and often leads to worse survival outcomes.
When the disease is identified at the localized stage, modern treatment methods can be highly effective. In fact, five-year survival rates can exceed 95% [1]. However, once the tumor spreads beyond the prostate, the situation changes. In particular, men diagnosed with metastatic prostate cancer face reduced survival rates. This is because the cancer often progresses despite hormonal therapy or chemotherapy. Traditional treatment options for advanced disease may help slow progression. However, they frequently come with significant side effects and limited long-term efficacy.
Recently, medical science has allowed to develop innovative options to treat prostate cancer. In particular, Lutetium 177 PSMA therapy has shown encouraging results in men with advanced and metastatic disease who have exhausted standard treatment lines. We want to invite prostate cancer patients and their families to read this article to learn how this approach can offer both improved survival and better quality of life.
The Principle of Lutetium 177 PSMA Therapy for Prostate Cancer
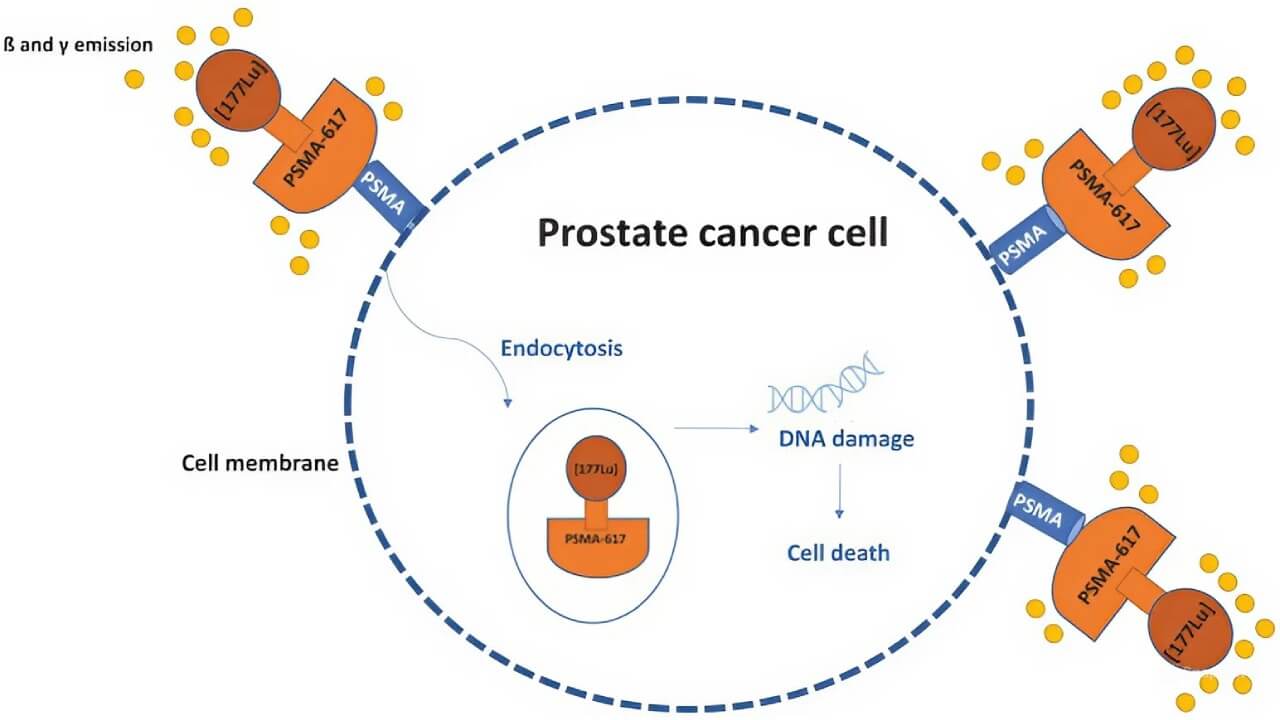
Lutetium 177 PSMA therapy (Lu-177 PSMA) is considered one of the most promising developments in nuclear medicine for men with advanced or metastatic prostate cancer. Traditional chemotherapy or hormonal treatments act on the whole body. In contrast, this therapy belongs to the class of radionuclide therapy, where radioactive isotopes are attached to molecules that seek out and destroy cancer cells [2, 3].
This approach is unique because it uses the prostate-specific membrane antigen (PSMA). This is a protein found in very high amounts on the surface of most prostate cancer cells, especially in aggressive or advanced stages of the disease [2]. Doctors can take advantage of this biological "flag" on tumor cells. By doing so, they can deliver targeted treatment directly where it is needed.
To get a more detailed explanation, we would like to invite you to watch this short video:
Targeting Prostate Cancer at the Cellular Level: Treatment with Lutetium-177 PSMA
How PSMA Targeting Works in Prostate Cancer
The Lutetium cancer treatment involves several steps:
PSMA Expression Assessment. As the research evidence shows, 80-95% of prostate cancers produce large amounts of PSMA. This makes it an excellent "target" for therapy.
Ligand Binding. Scientists have developed a small carrier molecule. It is called a ligand. It is designed to stick only to PSMA proteins on the cancer cell surface.
Radiolabeling. This ligand is chemically joined with Lutetium-177. Since it is a beta emitter, it can release radiation that kills cells.
Cell Destruction. After the Lu 177 PSMA compound is injected into a patient's vein, it circulates through the bloodstream. It finds the PSMA-positive tumor cells and attaches to them. Once bound, the isotope delivers tiny doses of radiation that destroy the malignant tissue [3-5].
In simple terms, the ligand acts like a "guided missile," PSMA is the "target," and Lutetium-177 is the "payload" that destroys the cancer.
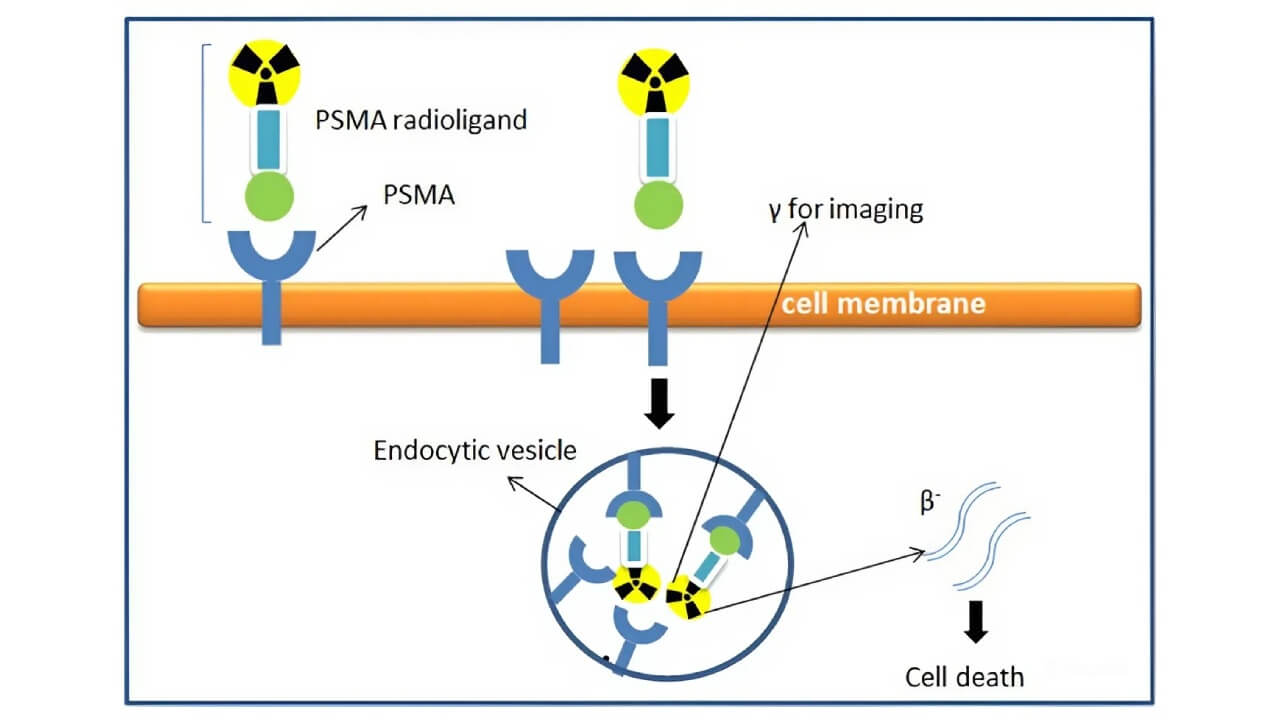
Mechanism of Action of Lutetium-177 in Prostate Cancer
Therapeutic Action. It releases beta particles that penetrate only 0.2-2 mm into the surrounding tissue. This short range is long enough to kill cancer cells. However, it is short enough to leave most nearby healthy cells untouched.
Diagnostic Action. At the same time, Lutetium-177 gives off a small amount of gamma radiation. This can be captured on a scan: doctors can see exactly where the drug has gone and how the cancer is responding [4, 5].
This combination of therapy and imaging is known as theranostics. It gives physicians two advantages at once. They can treat prostate cancer and check whether the treatment is working.
Advantages of Beta Radiation in Prostate Cancer
It is necessary to note that the type of radiation used is especially important. Beta radiation from Lutetium-177 has several advantages. First, because it penetrates only a few millimeters, it avoids harming healthy tissue further away from the tumor. Second, small, early metastatic deposits can still be destroyed. Third, in cases of bone metastases or liver involvement, Lu-177 PSMA therapy can be particularly valuable [4, 5].
Why It Is Effective Even in Metastatic Cases of Prostate Cancer
Lu-PSMA therapy has several strengths that make it successful even in late-stage disease. First, it does not just attack the primary prostate tumor but also finds and treats distant metastases throughout the body. Second, the therapy can be given in multiple rounds, with each cycle spaced several weeks apart. Moreover, Lu-PSMA is generally well-tolerated, with side effects that are milder and easier to manage [4, 5].
Goals of Treating Prostate Cancer with Lu-177 Therapy
The primary aim of Lu-PSMA therapy is to provide real and measurable benefits for men with advanced prostate cancer [2, 3]. Specifically, this modern therapy focuses on four main goals. First and foremost, for many prostate cancer patients, the most important outcome is living longer. Clinical trials have shown that men treated with Lu-PSMA survive several months longer compared to those on standard therapy [3-5]. Moreover, one of the biggest challenges in metastatic prostate cancer is that tumors can spread to many areas (e.g., bones, lymph nodes, or liver). However, Lu-PSMA is designed to seek out and destroy these hidden cancer cells wherever they are located [5].
Furthermore, living longer is important, but so is living better. Lu-PSMA delivers radiation only to cancer cells. As a result, patients often experience: less pain, especially bone pain; more energy and improved mobility; and fewer serious side effects compared to chemotherapy [4, 5]. For many prostate cancer patients, this improvement in daily life is just as valuable as survival.
Lastly, doctors track how well a treatment is working by measuring prostate-specific antigen (PSA) levels in the blood and monitoring tumor size on imaging. In healthy men, PSA levels are usually below 4 ng/mL; values between 4-10 ng/mL may suggest an increased risk of prostate cancer; levels above 10 ng/mL are more strongly associated with malignancy.
With Lu-PSMA therapy, strong biochemical responses are often seen, such as a significant drop in PSA levels. This reduction signals that the cancer cell population is shrinking. Smaller tumors can relieve symptoms, reduce pressure on nearby organs, and make subsequent treatments more effective [5, 6].
Preparation for PSMA Therapy for Prostate Cancer
Before starting Lu-PSMA therapy, prostate cancer patients go through a preparation process. This step ensures that the treatment will be safe. It also maximizes the chances of success.
Key Steps in Patient Preparation
Clinical evaluation. Only prostate cancer patients with confirmed PSMA-positive disease are eligible. This is verified through special scans (e.g., PSMA PET/CT). Patients with PSMA-negative scans are usually excluded from Lu-PSMA therapy; they may be offered alternative treatment options. A team of specialists reviews the patient's history, current condition, and test results. This teamwork ensures that no detail is overlooked.
Laboratory tests. Doctors check blood counts (to ensure bone marrow is strong enough), as well as liver and kidney function (since these organs help process and eliminate the drug). Urine analysis evaluates kidney performance, which is critical because the radioactive compound is cleared through the urinary system.
Imaging studies:
- PSMA PET/CT or SPECT scans map where the tumors are located and confirm PSMA expression.
- MRI or ultrasound provide additional detail about the size and spread of the tumor in the prostate or nearby organs.
- Scintigraph conducted before therapy to measure the overall disease burden.
Risk Minimization Measures
Doctors also take preventive steps to protect organs and reduce potential side effects:
| Measure | Purpose |
|---|---|
| Allergy test | Checks for hypersensitivity to the PSMA-ligand before infusion. |
| Renal function monitoring | Prevents kidney complications, since Lu-PSMA is cleared through the kidneys. |
| Hydration protocol | Encourages faster flushing of radioactive compounds from the body. |
| Cooling of salivary glands | Protects against dry mouth and salivary gland irritation, a common mild side effect. |
Dietary and Lifestyle Recommendations in Prostate Cancer
Moreover, preparation does not end in the hospital. Prostate cancer patients should avoid alcohol, fatty, and fried foods for a few days before therapy. They should also drink more fluids before and after therapy. Some drugs that affect kidney function may need to be paused. This is done under strict medical supervision to avoid risks.
How Lutetium Prostate Cancer Treatment Works
For men with metastatic prostate cancer, undergoing Lutetium 177 PSMA therapy is a structured and monitored process [3-5].
Hospital Admission and Setup
Most patients are admitted for 3-4 days. During this time, doctors review recent imaging and laboratory results to confirm readiness for therapy. Risk factors are assessed, and the patient has a detailed consultation about what to expect. Protective strategies (e.g., hydration protocols and salivary gland cooling) are explained, so patients know how Lutetium-177 PSMA side effects will be minimized.
Step-by-Step Administration
Intravenous Infusion. The actual treatment begins with a simple IV infusion of the radiopharmaceutical containing Lutetium 177 PSMA. Once in the bloodstream, the compound travels throughout the body, seeking out and attaching to prostate cancer cells that carry the PSMA marker.
Targeted Radiation Delivery. Once bound to the cancer, the drug emits beta particles that penetrate only 0.2-2 mm. As discussed earlier, this short range allows precise destruction of tumors while sparing surrounding healthy tissue.
Safety Measures. Patients are encouraged to drink fluids to help flush radioactive material safely. Ice packs or cooling devices are placed on the cheeks to reduce uptake in the glands and lower the risk of dry mouth. Antiemetics (to prevent nausea) and pain relievers are provided as needed.
Multiple Therapy Cycles in Prostate Cancer
Lu-PSMA therapy is delivered in cycles, each spaced 6-8 weeks apart. However, the exact number of cycles depends on how well the tumor responds and how well the patient tolerates treatment. Clinical studies show that prostate cancer patients who complete multiple cycles achieve better response rates and longer survival compared to those who stop after just one or two sessions [4, 5].
Side Effect Management in Prostate Cancer
Overall, Lutetium prostate cancer therapy is considered safe and much easier to tolerate than chemotherapy. Still, some side effects can occur, including fatigue (20-30%); xerostomia, or dry mouth, due to salivary gland uptake (10-20%); mild nausea (10-15%); and temporary drops in blood counts from bone marrow suppression (rare). These side effects are usually mild and manageable, and most resolve after treatment cycles end.
Comparison with External Beam Radiation Therapy in Prostate Cancer
As previously mentioned, External Beam Radiation Therapy (EBRT) is one of the most common types of radiation therapy used in prostate cancer treatment. It may seem unusual to compare EBRT with Lutetium 177 PSMA therapy, since they are used at different stages of prostate cancer. Specifically, EBRT is a localized treatment, often offered in earlier disease, while Lu-PSMA is a systemic radionuclide therapy used for advanced or metastatic prostate cancer [8]. However, placing the two side by side helps highlight what makes Lu-PSMA unique.
- External Beam Radiation Therapy (EBRT): Very effective for localized prostate cancer, but less suitable for widespread disease. Toxicity becomes a problem when trying to irradiate multiple metastases.
- Lutetium 177 PSMA Therapy: Works systemically – meaning it circulates through the body and targets both visible and microscopic metastases. It is also well tolerated, even in men who have already received extensive prior treatments.
This comparison demonstrates how the treatment paradigm in prostate cancer is evolving: from traditional, localized radiation approaches like EBRT to innovative systemic therapies such as Lu-PSMA, which can bring hope even in late stages of disease.
Efficiency Control and Follow-Up in Prostate Cancer Patients
The success of Lu-PSMA therapy for prostate cancer is not measured only by how a patient feels during treatment, but also by ongoing follow-up. This follow-up shows whether the therapy is providing effective metastasis control. It also helps doctors decide if more treatment cycles are needed or if it is time to consider alternative treatment options [4, 5].
Methods of Treatment Monitoring
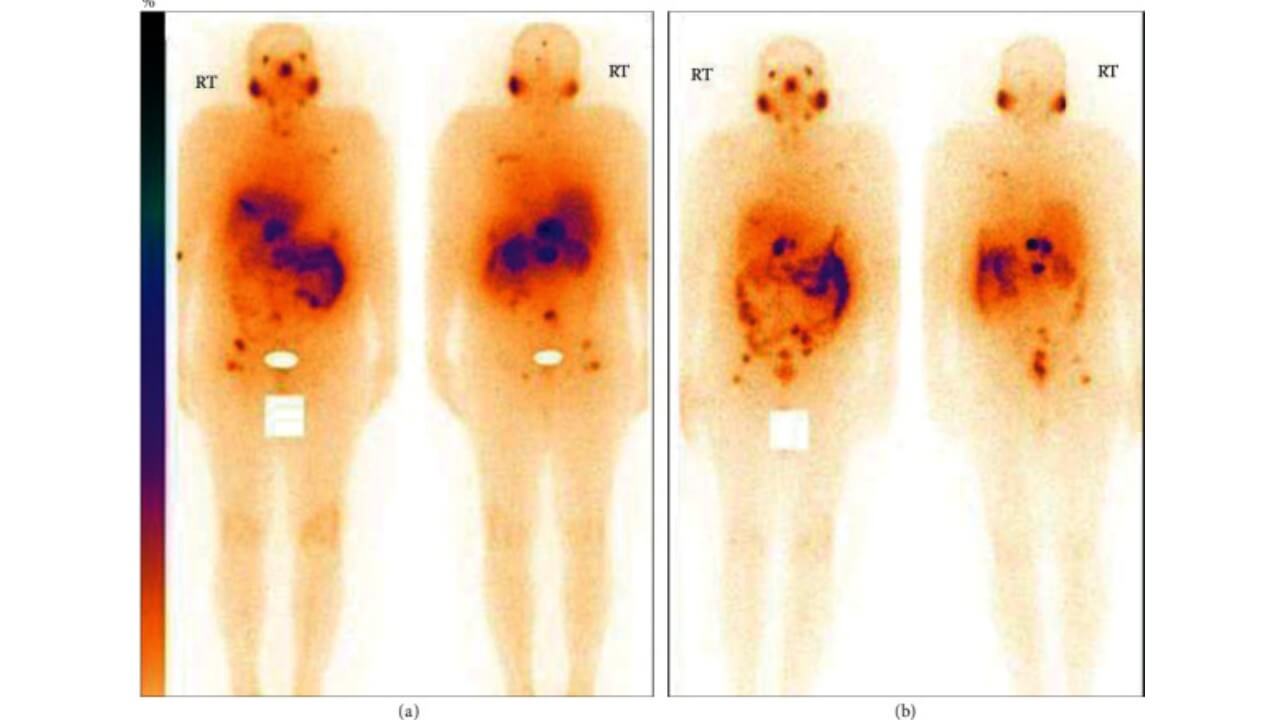
Imaging studies help evaluate therapy response. Specifically, scintigraphy/SPECT show how much of the radioligand has been absorbed by tumors and whether activity has decreased. PSMA PET/CT can detect even tiny deposits of disease, confirming whether cancer cells remain active. MRI or CT Scans provide detailed pictures of tumor size and anatomy, helping to track shrinkage and involvement of nearby organs.
Blood and urine tests complement imaging by providing biological evidence of response. For example, a drop of ≥50% in PSA is widely accepted as a strong indicator that the therapy is working. Regular checks of white blood cells, red blood cells, and platelets help catch temporary bone marrow suppression early. Since Lu-PSMA is cleared through these organs, ongoing monitoring ensures treatment remains safe.
Doctors also look at how the patient feels day to day. Many men report less bone pain, especially when metastases are present. Improved energy, mobility, and reduced fatigue are positive signs that therapy is improving quality of life.
Benefits of Prostate Cancer Treatment with Lutetium-177
The introduction of Lutetium 177 PSMA radioligand therapy has changed how men with metastatic prostate cancer and those with advanced disease are treated. Clinical studies from around the world have shown improved Lutetium-177 life expectancy for prostate cancer patients.
Improved Survival
For example, the VISION trial, published in the New England Journal of Medicine in 2021, studied 831 men with advanced, treatment-resistant prostate cancer. It found that prostate cancer patients receiving Lutetium 177 PSMA therapy lived a median of 15.3 months, compared with 11.3 months in those who continued standard care [9].
Strong Biological Response in Prostate Cancer
Similarly, the TheraP trial, published in The Lancet the same year, compared Lu-PSMA with a chemotherapy drug often used when other treatments fail. Among 200 prostate cancer patients, 66% of men treated with Lu-PSMA achieved a PSA decline of at least 50%, compared with only 37% in the chemotherapy group.
Effective Metastasis Control
A multicenter German study led by Rahbar and colleagues in 2017 included 145 prostate cancer patients with resistant disease. After Lu-PSMA therapy, about three out of four patients experienced either partial remission or stable disease [10].
Where Is It Best to Undergo Lutetium Therapy for Prostate Cancer?
Germany is widely recognized as one of the global leaders in Lutetium PSMA therapy. At the heart of the country's success are its Comprehensive Cancer Centers. For patients, choosing a Comprehensive Cancer Center means more than access to advanced technology. It means integrated care, access to innovation, and coordinated support.
Why Choose Germany for Lu-PSMA Therapy for Prostate Cancer?
German specialists were among the leaders of Lutetium PSMA, with thousands of patients treated since 2015. Many of the most important clinical trials have been led or co-authored by German teams.
Moreover, Lu-PSMA therapy is delivered within a network of specialists who also provide access to other treatment options like surgery, systemic therapy, or immunotherapy, ensuring prostate cancer care is never limited to a single approach.
Further, hospitals are equipped with advanced PET/CT scanners, dosimetry systems, and protective protocols that ensure precision and patient safety at every step.
In addition, many German centers support international patients with English-speaking coordinators, travel assistance, and hospital navigation.
Leading German Clinics for Lutetium 177 Therapy for Prostate Cancer
If you are considering therapy in Germany, here are some of the best-known hospitals:
| Hospital | Department | Key Features |
|---|---|---|
| Helios Hospital Berlin-Buch | Department of Nuclear Medicine | Led by Prof. Dr. Stefan Dresel. He is a nuclear medicine expert with more than 20 years of experience. This department specializes in both Lutetium 177 PSMA and Lu-DOTATOC therapy. |
| University Hospital Rechts der Isar, Munich | Department of Nuclear Medicine | Directed by Prof. Dr. Wolfgang Weber. He is a leader in radiation oncology and molecular imaging with more than 250 scientific publications. The department is internationally recognized for its role in clinical trials of radionuclide therapy. |
| University Hospital LMU Munich | Department of Nuclear Medicine | Headed by Prof. Dr. Peter Bartenstein. He brings over 35 years of experience and has authored more than 450 scientific works. This department is part of a Comprehensive Cancer Center, offering prostate cancer patients access not only to Lu-PSMA but also to clinical trials and research-driven care. |
| University Hospital Carl Gustav Carus, Dresden | Department of Nuclear Medicine | Directed by Prof. Dr. Jörg Kotzerke. He is a respected board member of the German Society for Nuclear Medicine (DGN). The hospital is known for its leadership in developing Lu-PSMA treatment for prostate cancer and for its international patient services. |
Each of these hospitals meets strict European quality standards and offers specialized prostate cancer programs for international patients. Regarding the cost of Lutetium 177 PSMA therapy, it typically ranges from €12,700 to €28,900. However, the final Lutetium cost depends on the treatment cycle and the patient's individual needs.
Prostate Cancer Patient Story: John Baker's Journey with Lutetium-177 in Germany
John Baker (Colorado, USA) was diagnosed with prostate cancer five years ago. After trying heat therapy and targeted radiation without success, his cancer spread beyond the prostate. Traditional treatment options like chemotherapy were not appealing. Moreover, in the United States, Medicare would not cover Lutetium 177 prostate cancer treatment.
John and his wife contacted Booking Health to find this treatment at specialized clinics in Germany. John tolerated the therapy well. Apart from mild tiredness the day after his first infusion, he reported no significant side effects. His results were also impressive. His PSA level dropped from 3 to nearly undetectable. Scans confirmed that bone metastases had disappeared.
"I would recommend it to anyone – not just for prostate cancer but for any cancer that qualifies for Lutetium 177 PSMA therapy. It was simple, and it worked." – John Baker.
When Medicare Said No: Colorado Man Finds Hope in German Cancer Therapy
A Medical Journey: Every Step of the Way with Booking Health
Choosing the right path for cancer treatment is never easy. Patients facing advanced prostate cancer or living with the challenges of metastatic prostate cancer often feel overwhelmed by the number of therapies, consultations, and decisions. In such moments, support from an experienced and trustworthy partner can make all the difference.
For more than 12 years, Booking Health has guided international patients treated for cancer through this difficult journey. Our mission is simple: to ensure that every person receives the most effective, innovative, and personalized care available – without unnecessary stress or hidden costs.
Every patient's story is unique. That is why our specialists begin by carefully reviewing your medical reports and analyzing all available treatment options. Based on this, we design a tailored program in collaboration with leading German hospitals. Whether you require surgical intervention, modern systemic therapy, or innovative solutions such as Lutetium 177 PSMA therapy, we ensure you are connected with the right experts in prostate cancer treatment.
Booking Health takes care of more than just medical scheduling. We provide:
- Assessment and analysis of medical reports before your trip.
- Development of a personalized prostate cancer care program in cooperation with German specialists.
- Selection of the most suitable clinic, ensuring access to the top Comprehensive Cancer Centers.
- Preparation of medical documents and their translation.
- Visa, flight, and accommodation support to make travel stress-free.
- Interpreter services and cultural guidance, so language barriers never stand in your way.
- Clinic coordination and on-site supervision, ensuring therapy and follow-up run smoothly.
- 24/7 personal coordinator, available for every question or concern.
- Transparent budgeting with fixed cost guarantees, so you never face unexpected charges.
- Ongoing follow-up care, even after you return home.
Health is life's most precious value. Entrusting it requires confidence and compassion. At Booking Health, we combine both the reliability of medical expertise with genuine care for each patient's well-being. For men with advanced or metastatic prostate cancer – or anyone seeking the highest standards of international cancer treatment – we stand by your side from the very first consultation to your safe return home.
Contact Booking Health today to begin your journey toward personalized, world-class prostate cancer treatment with Lutetium 177 PSMA in Germany.
Cancer Treatment Abroad: Patient Experiences with Booking Health
Frequently Asked Questions of Our Patients About Lutetium‑177 PSMA Therapy for Prostate Cancer
Send request for treatmentLutetium 177 PSMA therapy is a modern form of radionuclide therapy for men with metastatic or advanced prostate cancer. It uses PSMA targeting molecules linked to a radioactive beta emitter, which delivers radiation directly to cancer cells. This provides tumor destruction with minimal damage to healthy tissue.
Candidates are usually men with metastatic prostate cancer who no longer respond to standard treatments, have PSMA-positive disease on PET/CT, and maintain good kidney and liver function.
The therapy is given by IV infusion. Once in the body, the drug binds to PSMA receptors on cancer cells. The attached beta emitter releases short-range radiation, ensuring effective metastasis control with minimal damage to healthy tissue. The therapy is usually repeatable, with cycles every 6-8 weeks.
Compared to chemotherapy or external radiation, Lu-177 PSMA offers PSMA targeting for precision; minimal damage to healthy tissue; low side effects; repeatable cycles (3-6 sessions); proven effective metastasis control with survival benefits shown in major trials.
Germany leads in radionuclide therapy, with top centers including University Hospital LMU Munich, University Hospital Rechts der Isar, Munich, Helios Hospital Berlin-Buch. All these clinics combine medical oncology expertise and advanced imaging for international prostate cancer patients.
Choose treatment abroad, and you will get the best results for sure!
Authors:
This article was edited by medical experts, board-certified doctors Dr. Nadezhda Ivanisova, and Dr. Bohdan Mykhalniuk. For the treatment of the conditions referred to in the article, you must consult a doctor; the information in the article is not intended for self-medication!
Our editorial policy, which details our commitment to accuracy and transparency, is available here. Click this link to review our policies.
Source:
[1] Frontiers in Public Health. Prostate Cancer Incidence and Mortality: Global Status and Temporal Trends in 89 Countries From 2000 to 2019. https://www.frontiersin.org/journals/public-health/articles/10.3389/fpubh.2022.811044/full
[2] National Cancer Institute. For Advanced Prostate Cancer, Radiopharmaceutical Improves Survival. https://www.cancer.gov/news-events/cancer-currents-blog/2021/prostate-cancer-psma-radiopharmaceutical-vision
[3] Rien Ritawidya, Hendris Wongso, Nurmaya Effendi et al. Lutetium-177-Labeled Prostate-Specific Membrane Antigen-617 for Molecular Imaging and Targeted Radioligand Therapy of Prostate Cancer. Adv Pharm Bull. 2023 Apr 29;13(4):701–711. doi: 10.34172/apb.2023.079. [DOI] [PMC free article]
[4] Mohammad R Alam, Shashi B Singh, Shreeya Thapaliya et al. A Review of 177Lutetium-PSMA and 225Actinium-PSMA as Emerging Theranostic Agents in Prostate Cancer. Cureus. 2022 Sep 20;14(9):e29369. doi: 10.7759/cureus.29369. [DOI] [PMC free article]
[5] Isabel Heidegger, Claudia Kesch, Alexander Kretschmer et al. Biomarkers to personalize treatment with 177Lu-PSMA-617 in men with metastatic castration-resistant prostate cancer - a state of the art review. Ther Adv Med Oncol. 2022 Mar 5;14:17588359221081922. doi: 10.1177/17588359221081922. [DOI] [PMC free article]
[6] Raviteja Nanabala, Maroor Raghavan Ambikalmajan Pillai, Buvaneswari Gopal. Preparation of Patient Doses of [177Lu]Lu-DOTATATE and [177Lu]Lu-PSMA-617 with Carrier Added (CA) and No Carrier Added (NCA) 177Lu. Nucl Med Mol Imaging. 2022 Oct 7;56(6):313–322. doi: 10.1007/s13139-022-00778-y. [DOI] [PMC free article]
[7] Mohini Guleria, Jeyachitra Amirdhanayagam, Haladhar D Sarma et al. Preparation of 177Lu-PSMA-617 in Hospital Radiopharmacy: Convenient Formulation of a Clinical Dose Using a Single-Vial Freeze-Dried PSMA-617 Kit Developed In-House. Biomed Res Int. 2021 Nov 20;2021:1555712. doi: 10.1155/2021/1555712. [DOI] [PMC free article]
[8] BMC Cancer. Tolerability of Concurrent External Beam Radiotherapy and [177Lu]Lu-PSMA-617 for Node-Positive Prostate Cancer in Treatment Naïve Patients, Phase I Study (PROQURE-I Trial). https://bmccancer.biomedcentral.com/articles/10.1186/s12885-023-10725-5
[9] O Sartor, J de Bono, KN Chi, K Fizazi et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. Author manuscript; available in PMC: 2022 Mar 16. Published in final edited form as: N Engl J Med. 2021 Jun 23;385(12):1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article]
[10] Kambiz Rahbar, Hojjat Ahmadzadehfar, Clemens Kratochwil et al. German Multicenter Study Investigating 177Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. Journal of Nuclear Medicine January 2017, 58 (1) 85-90; [DOI]
Read:
Prostate cancer treatment with dendritic cells in Germany
A Comprehensive Guide to Getting Prostate Cancer Treatment
Comprehensive Guide to Stage 4 Prostate Cancer: Treatment Options
Article menu:
- The Principle of Lutetium 177 PSMA Therapy for Prostate Cancer
- Goals of Treating Prostate Cancer with Lu-177 Therapy
- Preparation for PSMA Therapy for Prostate Cancer
- How Lutetium Prostate Cancer Treatment Works
- Efficiency Control and Follow-Up in Prostate Cancer Patients
- Benefits of Prostate Cancer Treatment with Lutetium-177
- Where Is It Best to Undergo Lutetium Therapy for Prostate Cancer?
- Prostate Cancer Patient Story: John Baker's Journey with Lutetium-177 in Germany
- A Medical Journey: Every Step of the Way with Booking Health
- Frequently Asked Questions of Our Patients About Lutetium‑177 PSMA Therapy for Prostate Cancer
Don't know where to start?
Contact Booking Health