Prostate cancer remains the most widespread oncological pathology among men and a leading cause of cancer death worldwide. According to epidemiological data, PCa is the most frequently diagnosed cancer among men in more than half of the countries in the world (112 of 185 countries/territories), with an estimated 1.4 million new cases in 2020. The highest age-standardized incidence rates are seen in Northern and Western Europe, the Caribbean, Australia/New Zealand, North and South America, and Southern Africa. PCa is the leading cause of cancer death among men in a quarter of the world’s countries (48 of 185 countries), with an estimated 375,000 deaths in 2020 [1]. These numbers highlight the urgent need for innovative cancer therapy options and underline why advanced cancer treatment strategies are now in focus.
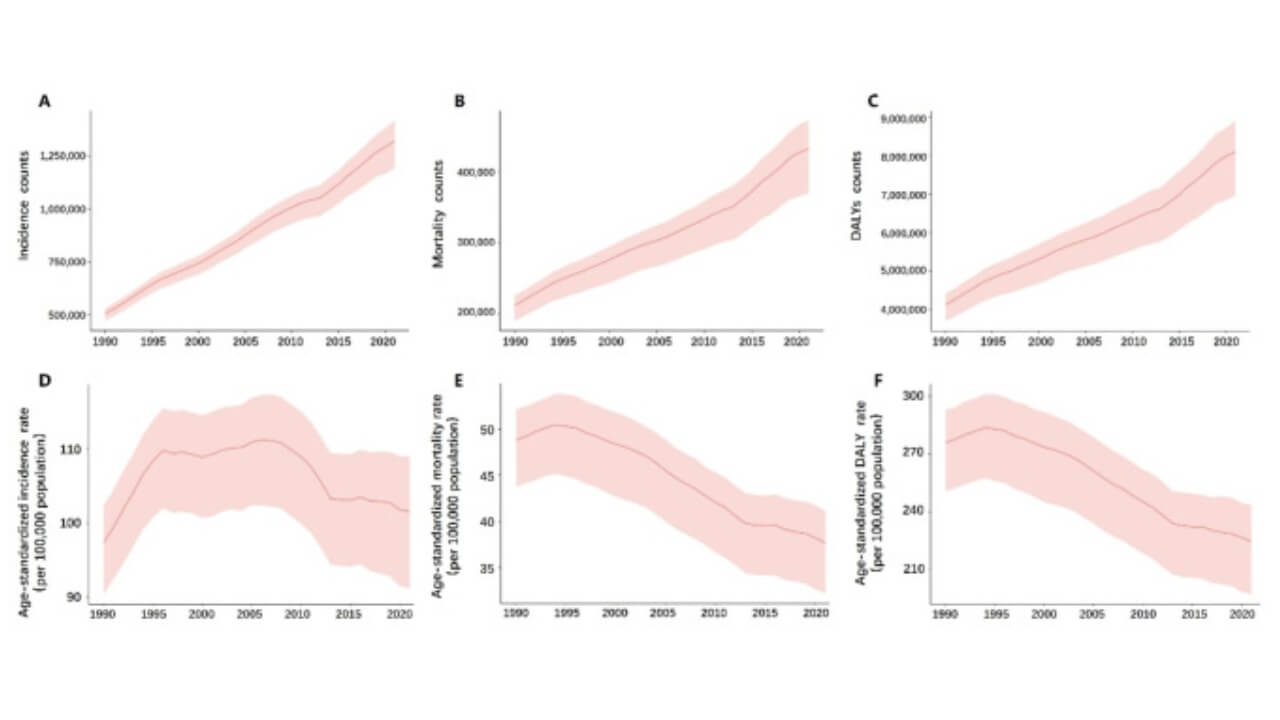
(A) Incidence
(B) Mortality
(C) DALY counts; and Age-standardized
(D) Incidence rate
(E) Mortality rate
(F) DALY rate
At the localized stages, traditional methods such as surgery, hormone therapy, or chemotherapy can be highly effective, but for patients with metastatic or castration resistant prostate cancer, opportunities for curative treatment are limited. This is where modern targeted alpha therapy is changing the landscape of oncology. Actinium-225 therapy is one of the most promising strategies, which is built on the capabilities of the use of the radioisotope Ac-225. In contrast to traditional beta-radiation, the technique utilizes high-energy particles from alpha emitters that have the effect of delivering a high radiation dose to cancer cells without impacting normal cells. The difference in these isotopes gives them a high targeting efficacy, where lethal DNA damage can be induced with a single dose and which would give high tumor control. Consequently, Actinium-225 has emerged as a pillar in the production of the next-generation cancer treatment for men with advanced prostate cancer.
Actinium-225: A Breakthrough Isotope in Targeted Alpha Therapy for Metastatic Prostate Cancer
Among modern isotopes used in innovative cancer treatment, Actinium-225 (Ac-225) has quickly gained recognition as one of the most powerful tools in nuclear medicine. This radionuclide uses high-energy alpha particles, unlike other previous alternatives, which use Lutetium-177, which is a beta radionuclide. Actinium-225 therapy is a game-changer for patients with metastatic prostate cancer through its capability to induce permanent DNA damage in cancer cells.
Over the past decade, targeted radionuclide therapy with Lutetium-177 has shown impressive results. An example is the TheraP clinical trial that compared Lutetium-177 PSMA therapy to chemotherapy among men with castration resistant prostate cancer. The findings were quite startling: over 66 percent of patients who took Lutetium responded to it, versus 37 percent of the patients in the chemotherapy group. Besides, Lutetium therapy had fewer adverse effects, which were severe and made it more usable and effective as compared to conventional methods. The second milestone was the VISION trial which enrolled more than 800 men with metastatic disease. The patients who were given Lutetium-177 along with standard care lived much longer and their quality of life was better than those who only received standard care. These outcomes firmly established PSMA-based therapies as a standard in advanced prostate cancer management.
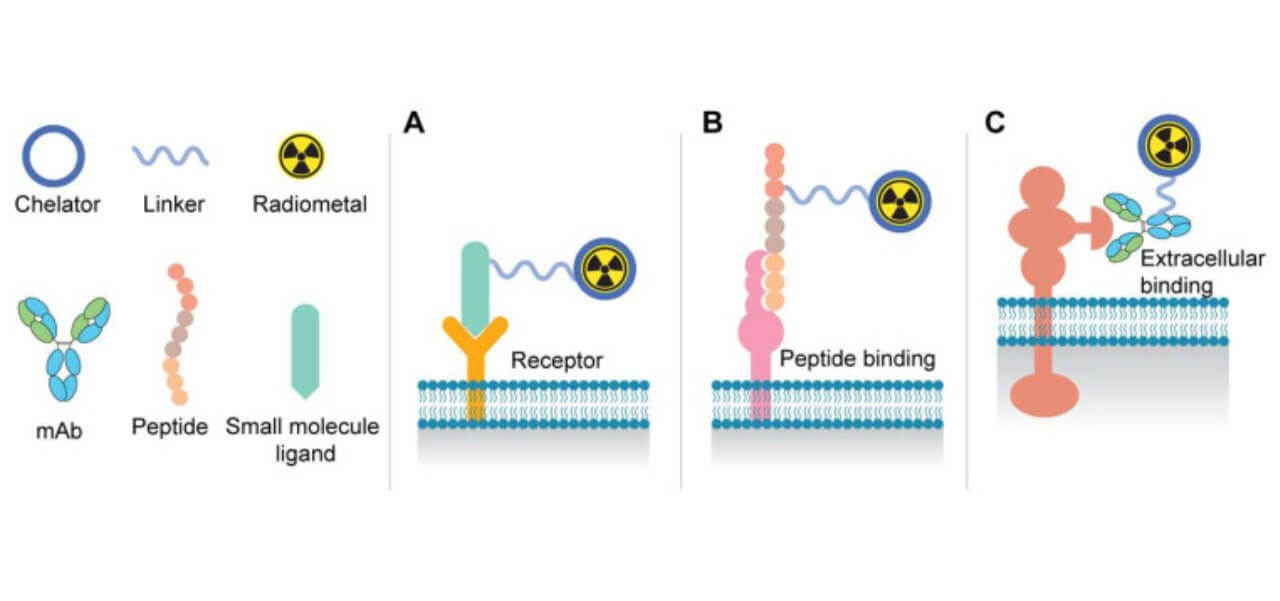
A. Binding of small molecule PSMA ligands to receptor active sites on the cell surface.
B. Representation of the use of peptide-based targeting agents.
C. Tumor-specific binding of antibodies to the extracellular domain of surface proteins.
Why Actinium-225 Isotopes Surpass Conventional Radionuclides in Metastatic Prostate Cancer
Despite these successes, not all patients respond to Lutetium-177. This shortcoming inspired the design of more powerful alpha emitter-based therapies. In contrast to the beta particles, which spread about several millimeters and can damage adjacent tissues, Ac-225 alpha particles can spread about a few diameters of cells. They are able to deliver an enormous radiation dose in a highly localized manner by virtue of their high-energy (LET of about 80 keV/μm) deposit. This is the exact action that allows them to destroy tumor cells selectively and spare normal cells nearby, which minimizes the possibility of collateral damage.
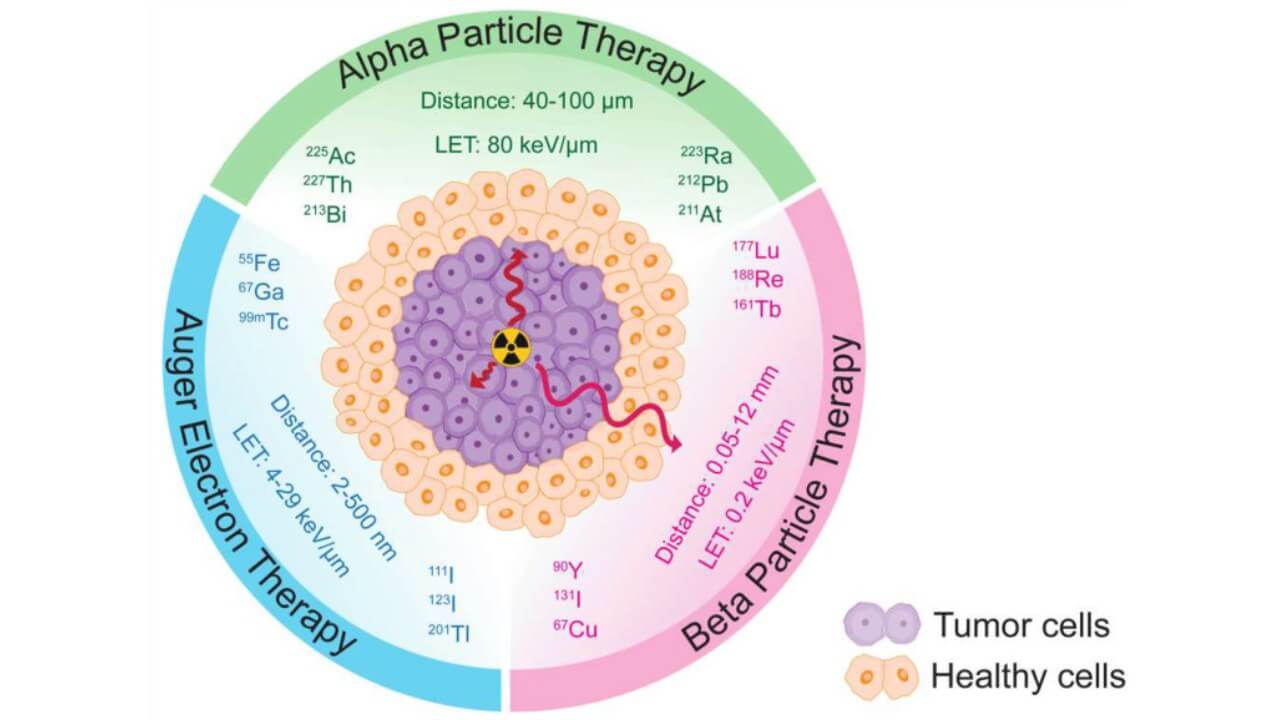
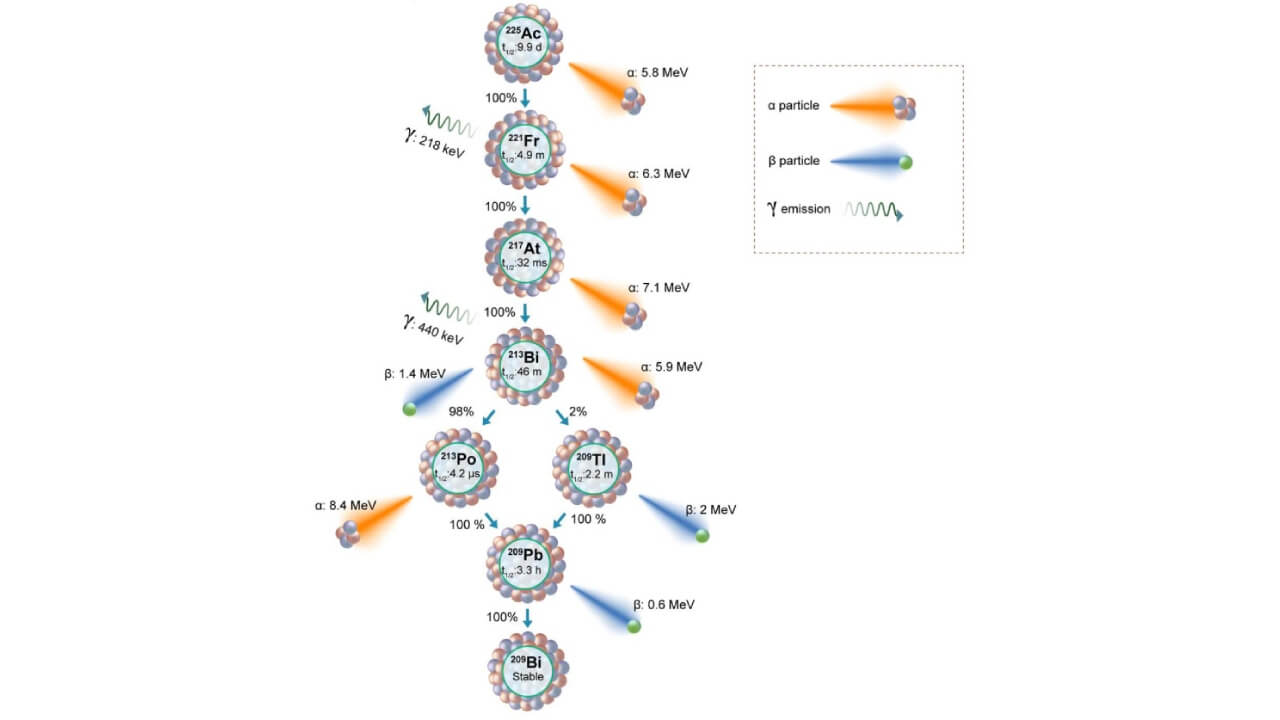
Every atom of Actinium-225 isotopes has a half-life of approximately 10 days and emits four powerful alpha particles. This is the most cytotoxic chain of alpha decay, and even a single dose of Actinium-225 could yield a significant tumor response. The therapy is extremely effective even when the patient has undergone chemotherapy, hormone therapy, or Lutetium-based therapies, due to its high efficiency in terms of targeting.
Clinical Potential and Flexibility of Actinium-225 in Metastatic Prostate Cancer
Emerging clinical trials highlight the remarkable benefits of Ac-225 isotopes. In early studies, men with advanced castration resistant prostate cancer who no longer responded to chemotherapy showed durable responses after several cycles of targeted alpha therapy. The unique mechanism of alpha radiation makes this possible: localized DNA destruction in cancer cells, minimal exposure to healthy cells, and lower rates of dose-limiting toxicity compared to older radionuclides [4].
Another important advantage is flexibility. Oncologists can apply Actinium-225 therapy as a primary strategy, as a combination with other cancer therapies, or as a sequential step after Lutetium-177. In other protocols, the Ac-225 isotopes are even thought over before chemotherapy, and they provide the patient with the benefits of nuclear medicine without the systemic toxicity. This adaptability underscores why Actinium-225 isotopes have become central to the development of next-generation cancer therapy for metastatic and castration resistant prostate cancer.
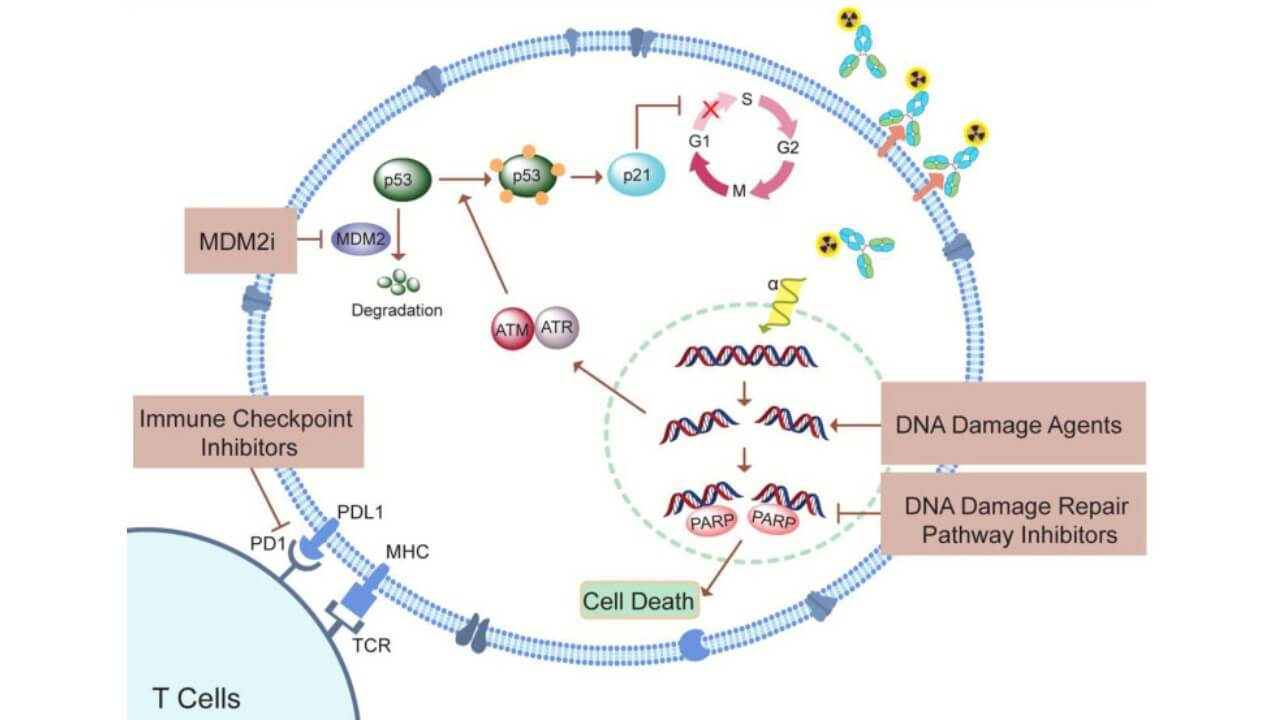
The Results of PSMA Therapy with Actinium-225 Isotope in Metastatic Prostate Cancer
The first clinical trials of PSMA expression–based therapy with Actinium-225 isotopes (Ac-225) have already shown impressive results, with measurable responses appearing within weeks after treatment. One of the most notable clinical outcomes is the rapid decline in PSA levels [5]. After Actinium-225 therapy, over 50% reduction in PSA has been documented in almost all patients, and in many German centers, the level even dropped below the detection threshold of 0.1 ng/ml. This demonstrates the high targeting efficiency of the method, where a carefully measured single dose of alpha emitters destroys a large number of cancer cells with remarkable precision.
PET/CT scans using PSMA as the follow-up in German hospitals show regression of metastatic lesions as well as shrinkage of the primary tumor. In contrast to conventional beta radiation, which can damage the surrounding tissues, high-energy alpha particles produced by Ac-225 isotopes deliver a localized dose of radiation to malignant foci and leave most normal cells intact. Thanks to Germany’s expertise in nuclear medicine, the therapy is not only highly effective but also safer in terms of lowering the risk of dose-limiting toxicity.
A major advantage of targeted alpha therapy with Actinium-225 isotopes in German clinics is the durability of response. Multi-year clinical trials demonstrate that patients with advanced castration resistant prostate cancer can achieve remissions lasting up to two years. Many international patients report improved survival and quality of life, highlighting the role of cancer therapy with isotopes as one of the most promising directions in modern oncology.
German studies also show striking results in chemotherapy-naive patients. More than 90% decline in PSA levels has been observed after Actinium-225 PSMA therapy, confirming that even those who failed hormone therapy or other conventional methods can still benefit. This proves the potential of Ac-225 isotopes as a first-line or rescue option in advanced cancer treatment.
Although production of isotopes is still limited and Actinium-225 therapy remains more expensive than Lutetium-177, Germany is one of the few countries where this treatment is available within structured development programs. With superior biological characteristics—shorter half-life, multiple alpha particles per decay, and unmatched targeting efficiency—Ac-225 therapy is expected to become a key innovation in German oncology, attracting patients worldwide.
| Survival rate | Disease progression | PSA levels | Side effects | Cost (EUR) |
|---|---|---|---|---|
| May vary greatly* | Remission up to 2 years | 50-90% | Xerostomia (dry mouth) | 17,000 - 28,000 per cycle |
*Data varies greatly depending on the previous treatment strategies, number of cycles administered, and patient health status.
How Will The Treatment for Metastatic Prostate Cancer be Carried Out?
In Germany, PSMA therapy with Actinium-225 isotopes (Ac-225) is carried out in specialized departments of nuclear medicine. The entire course of treatment, including the administration of the radiopharmaceutical and in-hospital monitoring, usually takes only 3–4 days. This short stay is sufficient to ensure that the radiation dose drops to a safe level, allowing the patient to return to normal life quickly. Such an approach reflects both the high precision and safety standards of German oncology centers, which attract patients from all over the world.
Preparatory Diagnostics and Protective Measures
The doctors conduct an extensive diagnostic program before treatment. It involves PSMA PET/CT that displays the spread of metastases, particularly to the spine and bones. Based on these results, the oncologist plans the procedure. Preparative precautions are also implemented: the insertion of the venous catheters is made to deliver the radiopharmaceutical safely, the cooling of the salivary glands is implemented to minimize the uptake of the radionuclide, and the intravenous saline protects the kidneys. Thanks to these steps, side effects are minimized, and the method achieves excellent targeting efficiency.
Administration of The Radiopharmaceutical
The infusion itself takes less than 20 seconds. Once injected, Actinium-225 isotopes spread through the bloodstream and bind to cancer cells that have PSMA expression. This ensures that the alpha emitters deliver high-energy alpha particles directly into malignant tissues, sparing healthy cells. Distribution of the radionuclide is monitored with scintigraphy, which confirms both tumor targeting and the reduction of metastases. According to clinical experience in Germany, the procedure is generally well tolerated. Only mild reactions, such as nausea or dry mouth, are reported.
Treatment Cycles and Long-term Outcomes in Metastatic Prostate Cancer
To achieve the most favorable result, Actinium-225 therapy is performed in several sessions. This approach prolongs the exposure of cancer cells to the alpha radiation and improves long-term outcomes. The number of cycles is determined individually, depending on the stage of prostate cancer, the presence of castration resistant disease, and the patient’s overall condition. German clinical trials have confirmed that repeating sessions significantly increases remission rates and strengthens disease control. German clinical trials have confirmed that repeating sessions significantly increases remission rates with only mild dose limiting toxicity often associated with chemo or conventional beta radiation.
Where can you undergo PSMA therapy with Actinium-225 Isotopes for Metastatic Prostate Cancer?
PSMA therapy with Actinium-225 isotopes (Ac-225) is actively performed in Germany, the home of this innovative cancer therapy. German specialists, in close cooperation with leading research centers, developed this targeted alpha therapy, evaluated its effectiveness in clinical trials, and successfully introduced it into routine practice. German oncologists offer not only highly effective but also safe care due to their experience in the treatment of cancer cells with high-energy alpha particles, their expertise in nuclear medicine, and their experience in working with alpha emitters.
Leading German Clinics for Metastatic Prostate Cancer Treatment
The patients interested in receiving Actinium-225 treatment can find many prestigious hospitals in Germany, such as:
- Department of Nuclear Medicine, University Hospital Carl Gustav Carus Dresden
- Department of Nuclear Medicine, University Hospital Erlangen
- Department of Nuclear Medicine, University Hospital Duesseldorf
Such hospitals provide guided programs of PSMA expression-based therapies. Actinium-225 isotopes can be used efficiently and safely to administer alpha radiation to cancer cells only, so that healthy cells are not exposed.
How Booking Health Supports International Patients with Metastatic Prostate Cancer
The registration process for Actinium-225 isotope therapy in Germany can be complex. Booking Health simplifies it for patients from all over the world. The company maintains a high level of coordination between the point of clinic choice to returning home, and takes care of all medical and organizational arrangements.
Services include:
- Applying for invitations to get visas to receive treatment on time
- Making appointments when it is convenient
- Preparation of initial tests, such as PSMA PET/CT
- Pick-up and drop-off at the airport
- Interpreter services and an individual medical coordinator
- Up to 200,000 euros of health insurance against complications
- Medical report and recommendation writing and translation
- Assistance in further contact with doctors, remote consultations
Advantages of Undergoing Treatment of Metastatic Prostate Cancer in Germany
When opting to pursue the treatment of Actinium-225 in Germany, patients will benefit by having access to the new clinical trials, the latest targeted alpha therapies, and new approaches in cancer treatment. German clinics are set up to administer Ac-225 isotopes with very high levels of target efficiency so that high-energy alpha particles strike the cancer cells in a very specific manner. International patients no longer need to wait for these techniques to be available in their home countries. With Booking Health, they can receive personalized treatment, maximize the therapeutic effect of Actinium-225 therapy, and save precious time while benefiting from world-class medical expertise.
A Medical Journey: Every Step of the Way With Booking Health
Finding the best treatment strategy for your clinical situation is a challenging task. Being already exhausted from multiple treatment sessions, having consulted numerous specialists, and having tried various therapeutic interventions, you may be lost in all the information given by the doctors. In such a situation, it is easy to choose a first-hand option or to follow standardized therapeutic protocols with a long list of adverse effects instead of selecting highly specialized innovative treatment options.
To make an informed choice and get a personalized cancer management plan, which will be tailored to your specific clinical situation, consult medical experts at Booking Health. Being at the forefront of offering the latest medical innovations for already 12 years, Booking Health possesses solid expertise in creating complex management programs in each individual case. As a reputable company, Booking Health offers personalized prostate cancer treatment plans with direct clinic booking and full support at every stage, from organizational processes to assistance during treatment. We provide:
- Assessment and analysis of medical reports
- Development of the medical care program
- Selection of a suitable treatment location
- Preparation of medical documents and forwarding to a suitable clinic
- Preparatory consultations with clinicians for the development of medical care programs
- Expert advice during the hospital stay
- Follow-up care after the patient returns to their native country after completing the medical care program
- Taking care of formalities as part of the preparation for the medical care program
- Coordination and organization of the patient's stay in a foreign country
- Assistance with visas and tickets
- A personal coordinator and interpreter with 24/7 support
- Transparent budgeting with no hidden costs
Health is an invaluable aspect of our lives. Delegating management of something so fragile yet precious should be done only to experts with proven experience and a reputation. Booking Health is a trustworthy partner who assists you in pursuing stronger health and a better quality of life. Contact our medical consultant to learn more about the possibilities of personalized treatment with innovative methods for prostate cancer with leading specialists in this field.
Advanced Cancer Treatment: Patient Success Stories with Booking Health
FAQ: Actinium-225 PSMA Therapy for Metastatic Prostate Cancer
Send request for treatmentActinium-225 isotopes PSMA therapy is an advanced cancer therapy that uses targeted alpha therapy to treat metastatic castration resistant prostate cancer. It binds Actinium-225 isotopes to molecules targeting PSMA on cancer cells, delivering high-energy alpha particles for precise tumor destruction. This method represents a new development in cancer treatment with controlled radiation exposure.
The therapy introduces alpha particles of high energy straight into the PSMA-expressing cancer cells, resulting in the formation of double-stranded DNA breaks but causing minimal destruction to healthy cells. With short range and high radiation potency of alpha emitters, this Actinium-225 isotope therapy ensures that it works as much as possible within one dose. The half-life of the isotopes can enable constant exposure to tumors, which increases the overall effectiveness of the treatment of cancer.
This is an intravenous infusion of Actinium-225 in nuclear medicine facilities. A combination strategy is usually given to patients, using one or several sessions, with close attention to dose-limiting toxicity and response to treatment. German facilities are advanced in the manufacturing of high-quality isotopes and organizing regimens of effective and safe cancer therapy.
Clinical trials indicate PSA suppression in more than 75 percent of the patients and massive tumor regression. Progression-free survival is much better in castration resistant prostate cancer. The accuracy of the Actinium-225 isotopes of the alpha radiation is the focus of targeted therapy in treating cancer and minimizing poisoning.
It can be accessed in Germany as well as other countries that have specialized nuclear medicine centers. Medical tourism operators ensure the appropriate examination of patients (PSMA PET/CT) before treatment. The handling of Actinium-225 isotopes is done with a lot of care in order to increase the delivery of radiation and ensure the best cancer therapy.
In Germany, it costs between €15,000 and €29,000, depending on location, production of the Actinium-225 isotopes, and the resources of the hospital. The high treatment potential, the accuracy of alpha radiation, and systematic clinical development are worth the investment.
The therapy offers highly selective cancer therapy, killing cancer cells with high-energy alpha particles while sparing healthy cells. Even patients resistant to standard approaches benefit. With controlled radiation, repeated sessions, and minimal dose limiting toxicity, Actinium-225 isotopes therapy represents a breakthrough in advanced cancer treatment.
Actinium-225 isotopes release more potent alpha radiation and maintain a favorable half-life for tumor targeting. Unlike Lutetium-177, it can succeed in previously resistant castration resistant prostate cancer cases. German clinical trials confirm superior cancer therapy outcomes, combining multiple alpha emitters in a single combination treatment approach.
Treatment is generally well tolerated. Mild reactions may include fatigue, nausea, or dry mouth. Severe complications are rare thanks to German protocols controlling radiation, dose-limited toxicity, and careful production of Actinium-225 isotopes. This makes the therapy a safe and precise cancer treatment option.
How does Actinium-225 isotopes PSMA therapy impact life expectancy for patients with metastatic prostate cancer?
Patients receiving Actinium-225 isotopes therapy often see prolonged survival and improved quality of life. In castration resistant prostate cancer, repeated single doses of high-energy alpha particles contribute to tumor regression. German development programs ensure consistent radiation dosing, making this cancer therapy a highly effective long-term treatment.
Choose treatment abroad and you will get the best results for sure!
The Booking Health portal presents 41 German clinics specializing in PSMA therapy with Actinium-225
See the interview for more information:
RADIONUCLIDE TREATMENT OF PROSTATE CANCER IN GERMANY – interview with Prof. Dr. med. Stefan Dresel
Authors:
This article was edited by medical experts, board-certified doctors Dr. Nadezhda Ivanisova, and Dr. Bohdan Mykhalniuk. For the treatment of the conditions referred to in the article, you must consult a doctor; the information in the article is not intended for self-medication!
Our editorial policy, which details our commitment to accuracy and transparency, is available here. Click this link to review our policies.
Sources:
[1] Oskar Bergengren, Kelly R Pekala, Konstantina Matsoukas et al. 2022 Update on Prostate Cancer Epidemiology and Risk Factors—A Systematic Review. Eur Urol. Author manuscript; available in PMC: 2024 Aug 1. Published in final edited form as: Eur Urol. 2023 May 16;84(2):191–206. doi: 10.1016/j.eururo.2023.04.021. [DOI] [PMC free article]
[2] Feifan Chu, Lumin Chen, Qing Guan, Zujie Chen et al. Global burden of prostate cancer: age-period-cohort analysis from 1990 to 2021 and projections until 2040. World J Surg Oncol. 2025 Mar 20;23(1):98. doi: 10.1186/s12957-025-03733-1. [DOI] [PubMed]
[3] Anil P Bidkar, Luann Zerefa, Surekha Yadav, Henry F VanBrocklin, Robert R Flavell. Actinium-225 targeted alpha particle therapy for prostate cancer. Theranostics. 2024 May 11;14(7):2969–2992. doi: 10.7150/thno.96403. [DOI] [PMC free article]
[4] Girish Kumar Parida, Raj Abhisek Panda, Komal Bishnoi, Kanhaiyalal Agrawal. Efficacy and Safety of Actinium-225 Prostate-Specific Membrane Antigen Radioligand Therapy in Metastatic Prostate Cancer: A Systematic Review and Metanalysis. Med Princ Pract. 2023 May 29;32(3):178–191. doi: 10.1159/000531246. [DOI] [PMC free article]
[5] Gaia Ninatti, Pietro Scilipoti, Cristiano Pini et al. Time for action: actinium-225 PSMA-targeted alpha therapy for metastatic prostate cancer - a systematic review and meta-analysis. Theranostics. 2025 Feb 20;15(8):3386–3399. doi: 10.7150/thno.106574. [DOI] [PMC free article]
Read:
Revolutionary Immunotherapy Treatments for Prostate Cancer in Germany
Article menu:
- Actinium-225: A Breakthrough Isotope in Targeted Alpha Therapy for Metastatic Prostate Cancer
- The Results of PSMA Therapy with Actinium-225 Isotope in Metastatic Prostate Cancer
- How Will The Treatment for Metastatic Prostate Cancer be Carried Out?
- Where can you undergo PSMA therapy with Actinium-225 Isotopes for Metastatic Prostate Cancer?
- A Medical Journey: Every Step of the Way With Booking Health
- FAQ: Actinium-225 PSMA Therapy for Metastatic Prostate Cancer
Don't know where to start?
Contact Booking Health